| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
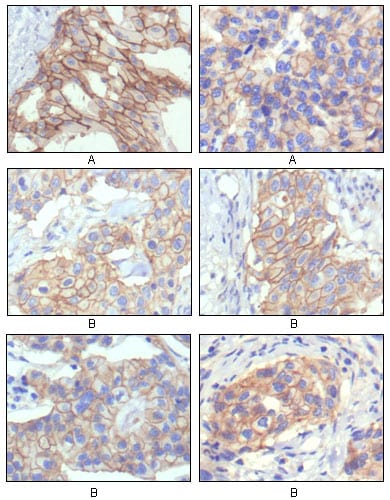
| IHC | 1/200 - 1/1000 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/10000 | Human,Mouse,Rat |
| Aliases | HER-2, C-erB-2, erbB-2 |
| Entrez GeneID | 2064 |
| clone | 9B9D8 |
| Host/Isotype | Mouse IgG2b |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Purified recombinant fragment of HER-2 expressed in E. Coli. |
| Formulation | Ascitic fluid containing 0.03% sodium azide. |
+ +
以下是3-4条关于HER-2抗体的代表性文献概览(按研究时间顺序):
---
1. **文献名称**: *Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2*
**作者**: Slamon, D. J., et al.
**摘要**: 该研究(2001年)首次证明抗HER-2单抗曲妥珠单抗(Trastuzumab)联合化疗可显著延长HER-2阳性转移性乳腺癌患者的总生存期和无进展生存期,奠定了HER-2靶向治疗的基础。
---
2. **文献名称**: *Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer*
**作者**: Baselga, J., et al.
**摘要**: 2012年发表的CLEOPATRA试验表明,帕妥珠单抗(Pertuzumab)联合曲妥珠单抗和多西他赛一线治疗HER-2阳性转移性乳腺癌,较单用曲妥珠单抗显著延长患者生存期,验证了双重HER-2阻断的临床优势。
---
3. **文献名称**: *Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer*
**作者**: Verma, S., et al.
**摘要**: EMILIA试验(2012年)显示,抗体药物偶联物T-DM1(曲妥珠单抗-美坦新偶联物)对比传统化疗方案,可显著改善HER-2阳性晚期乳腺癌患者的生存并降低毒性,推动了ADC药物在HER-2治疗中的应用。
---
4. **文献名称**: *Antibody Drug Conjugate Trastuzumab Deruxtecan in HER2-Positive Breast Cancer with Brain Metastases*
**作者**: Modi, S., et al.
**摘要**: 2020年DESTINY-Breast01试验证实,新型ADC药物德喜曲妥珠单抗(Trastuzumab Deruxtecan, T-DXd)对HER-2阳性乳腺癌伴脑转移患者具有显著疗效,突破了传统抗体对中枢神经系统转移的治疗局限。
---
**注**:以上文献均发表于《新英格兰医学杂志》(*NEJM*)等顶级期刊,涵盖HER-2靶向治疗的经典抗体、联合疗法及新型ADC药物进展。
HER-2 (Human Epidermal Growth Factor Receptor 2), also known as ERBB2. is a transmembrane tyrosine kinase receptor involved in regulating cell proliferation, survival, and differentiation. Overexpression or amplification of HER-2 occurs in 15-20% of breast cancers and some gastric, ovarian, and lung cancers, driving aggressive tumor growth and poor prognosis. This molecular alteration makes HER-2 a critical therapeutic target.
HER-2-targeted antibodies, such as trastuzumab (Herceptin®) and pertuzumab (Perjeta®), bind specifically to HER-2 extracellular domains, inhibiting downstream signaling pathways like PI3K/AKT and MAPK. Trastuzumab, the first FDA-approved HER-2 antibody (1998), also recruits immune cells to induce antibody-dependent cellular cytotoxicity (ADCC). These therapies revolutionized treatment for HER-2-positive cancers, significantly improving survival rates. However, primary or acquired resistance remains a challenge, prompting development of antibody-drug conjugates (ADCs) like trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd), which deliver cytotoxic payloads directly to cancer cells.
Diagnostically, HER-2 status is assessed via immunohistochemistry (IHC) for protein expression and fluorescence in situ hybridization (FISH) for gene amplification, guiding treatment decisions. Ongoing research focuses on combination therapies, novel ADCs, and strategies to overcome resistance, solidifying HER-2 antibodies as cornerstones of precision oncology.
×