首页 / 产品 / 蛋白 / 细胞因子、趋化因子与生长因子
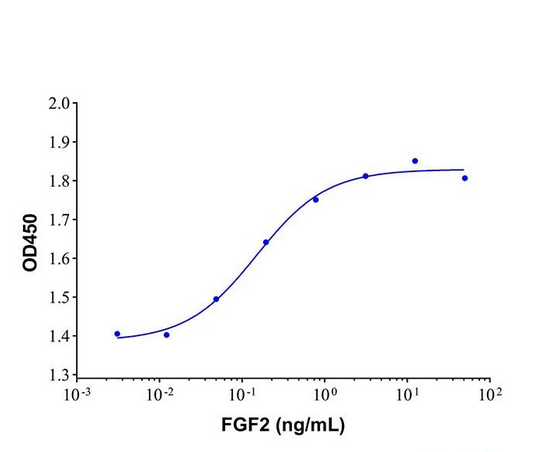
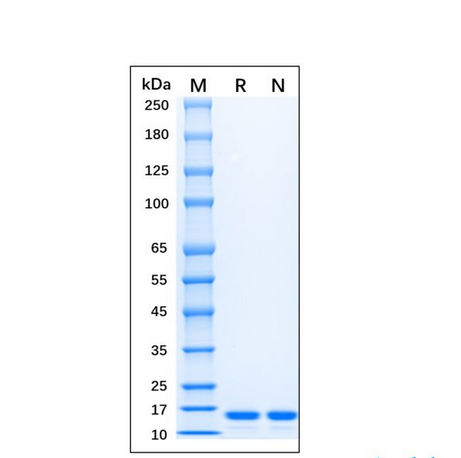
| 纯度 | >95%SDS-PAGE. |
| 种属 | Human |
| 靶点 | FGF2 |
| Uniprot No | P09038 |
| 内毒素 | < 0.01EU/μg |
| 表达宿主 | E.coli |
| 表达区间 | 135-288aa |
| 氨基酸序列 | AAGSITTLP ALPEDGGSGA FPPGHFKDPK RLYCKNGGFF LRIHPDGRVD GVREKSDPHI KLQLQAEERG VVSIKGVCAN RYLAMKEDGR LLASKCVTDE CFFFERLESN NYNTYRSRKY TSWYVALKRT GQYKLGSKTG PGQKAILFLP MSAKS |
| 预测分子量 | 17 kDa |
| 蛋白标签 | His tag N-Terminus |
| 缓冲液 | PBS, pH7.4, containing 0.01% SKL, 1mM DTT, 5% Trehalose and Proclin300. |
| 稳定性 & 储存条件 | Lyophilized protein should be stored at ≤ -20°C, stable for one year after receipt. Reconstituted protein solution can be stored at 2-8°C for 2-7 days. Aliquots of reconstituted samples are stable at ≤ -20°C for 3 months. |
| 复溶 | Always centrifuge tubes before opening.Do not mix by vortex or pipetting. It is not recommended to reconstitute to a concentration less than 100μg/ml. Dissolve the lyophilized protein in distilled water. Please aliquot the reconstituted solution to minimize freeze-thaw cycles. |
以下是3篇与FGF2重组蛋白相关的代表性文献摘要:
---
1. **文献名称**:*Isolation and characterization of acidic and basic fibroblast growth factor*
**作者**:Gospodarowicz D. et al.
**摘要**:该研究首次从牛垂体中分离纯化出碱性成纤维细胞生长因子(FGF2),证实其能促进血管内皮细胞和成纤维细胞的增殖,并建立了早期重组表达技术,为后续功能研究奠定基础。
---
2. **文献名称**:*Crystal structure of fibroblast growth factor 2 in complex with heparin sulfate*
**作者**:Zhang Y. et al.
**摘要**:通过X射线晶体学解析FGF2与硫酸肝素复合物的三维结构,揭示了FGF2与受体结合的关键结构域及硫酸肝素在稳定蛋白构象和信号传递中的作用。
---
3. **文献名称**:*Recombinant human FGF-2 for chronic wound healing: A phase I clinical trial*
**作者**:Akita S. et al.
**摘要**:一项临床试验表明,局部应用重组FGF2可显著加速糖尿病足溃疡和烧伤创面的愈合,其机制涉及促进血管新生和上皮再生,证实其临床应用潜力。
---
4. **文献名称**:*FGF2 maintains self-renewal of human embryonic stem cells*
**作者**:Chen C. et al.
**摘要**:研究发现重组FGF2通过激活MAPK/ERK信号通路,支持人胚胎干细胞(hESC)的长期自我更新,为无血清干细胞培养基的开发提供理论依据。
---
这些文献涵盖了FGF2的基础机制、结构解析及转化应用,可根据具体研究方向进一步扩展。
Fibroblast Growth Factor 2 (FGF2), also known as basic fibroblast growth factor (bFGF), is a multifunctional protein belonging to the FGF family, first identified in the 1970s for its mitogenic and angiogenic properties. It plays a critical role in regulating cell proliferation, differentiation, survival, and migration across diverse biological processes, including embryonic development, tissue repair, and angiogenesis. Structurally, FGF2 is a heparin-binding protein composed of 155–196 amino acids (depending on isoforms) and signals through tyrosine kinase FGF receptors (FGFRs) in complex with heparan sulfate proteoglycans (HSPGs).
Recombinant FGF2 is produced using genetic engineering techniques, typically expressed in *E. coli* or mammalian cell systems to ensure high purity and bioactivity. Its production enables standardized research and therapeutic applications, overcoming limitations of native FGF2 extraction, such as low yield and contamination risks.
In research, recombinant FGF2 is widely used to maintain stem cell pluripotency, expand primary cells *in vitro*, and model tissue regeneration. Clinically, it has been explored for wound healing, cardiovascular repair, and neurodegenerative therapies, though challenges like short half-life, instability, and off-target effects persist. Recent advances in delivery systems (e.g., biomaterial carriers) and engineered variants aim to enhance its stability and specificity.
Despite its therapeutic potential, safety concerns, including tumorigenic risks due to pro-angiogenic activity, necessitate careful dose optimization and long-term studies. Ongoing research focuses on balancing efficacy with safety, positioning recombinant FGF2 as a pivotal tool in regenerative medicine and drug development.
×