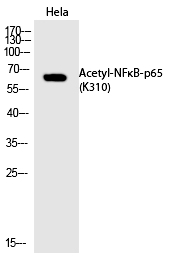
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/100-1/300 | Human,Mouse,Rat |
| ICC | 1/200-1/1000 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/40000 | Human,Mouse,Rat |
| Aliases | RELA; NFKB3; Transcription factor p65; Nuclear factor NF-kappa-B p65 subunit; Nuclear factor of kappa light polypeptide gene enhancer in B-cells 3 |
| Entrez GeneID | 5970; |
| WB Predicted band size | 65kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse |
| Immunogen | Synthesized peptide derived from human NFκB-p65 around the acetylation site of K310. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是3篇涉及NFκB-p65 (Acetyl-Lys310)抗体的代表性文献摘要:
1. **"Acetylation of RelA at lysine 310 is essential for NF-κB-dependent gene expression"**
*Chen L, Fischle W, Verdin E, Greene WC*
该研究通过Western blot和ChIP实验证明,p300介导的Lys310乙酰化是NF-κB转录激活的关键步骤,使用特异性抗体验证了乙酰化修饰在炎症基因表达中的作用。
2. **"Post-activation turn-off of NF-κB-dependent transcription is regulated by acetylation of p65"**
*Kiernan R, Brès V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, et al.*
研究发现Lys310乙酰化通过HDAC3去乙酰化调控NF-κB的转录关闭,抗体用于免疫荧光和免疫共沉淀实验,揭示了乙酰化动态平衡对炎症反应终止的机制。
3. **"Proinflammatory cytokines induce NF-κB-dependent DNA hyperacetylation through p65 acetylation at Lys310"**
*Luo JL, Kamata H, Karin M*
该文献利用乙酰化特异性抗体进行染色质分析,证实TNFα刺激下Lys310乙酰化促进促炎基因启动子的组蛋白超乙酰化,驱动肿瘤微环境中的炎症反应。
4. **"Site-specific phosphorylation-ubiquitination crosstalk regulates NF-κB signaling"**
*Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T*
研究通过免疫沉淀和Western blot证明DNA损伤后,Lys310乙酰化与磷酸化协同调控p65核转位,抗体用于检测电离辐射后乙酰化修饰的动态变化。
The NFκB-p65 (Acetyl-Lys310) antibody is a specialized tool used to detect the acetylated form of the NF-κB p65 subunit at lysine residue 310. NF-κB (Nuclear Factor kappa-light-chain-enhancer of activated B cells) is a transcription factor family critical for regulating immune responses, inflammation, cell survival, and proliferation. The p65 subunit (RelA) is a key component of the canonical NF-κB pathway, which is typically sequestered in the cytoplasm by inhibitory proteins (IκB) until activated by stimuli such as cytokines (e.g., TNF-α, IL-1β), pathogens, or stress signals. Upon activation, IκB is degraded, allowing p65 to translocate to the nucleus and initiate target gene transcription.
Acetylation of p65 at Lys310 is a post-translational modification that modulates its transcriptional activity. This modification, mediated by histone acetyltransferases (e.g., p300/CBP), enhances DNA binding affinity and promotes interactions with coactivators while reducing binding to IκBα, thereby prolonging nuclear retention. The acetylation status of Lys310 is thus pivotal in fine-tuning NF-κB-dependent gene expression. The NFκB-p65 (Acetyl-Lys310) antibody enables researchers to study this regulatory mechanism in contexts like inflammation, cancer, and immune disorders. It is widely used in techniques such as Western blotting, immunoprecipitation, and immunofluorescence to assess acetylated p65 levels, providing insights into pathway activation and therapeutic targeting. Specificity for the acetylated epitope ensures accurate detection, distinguishing it from non-modified p65.
×