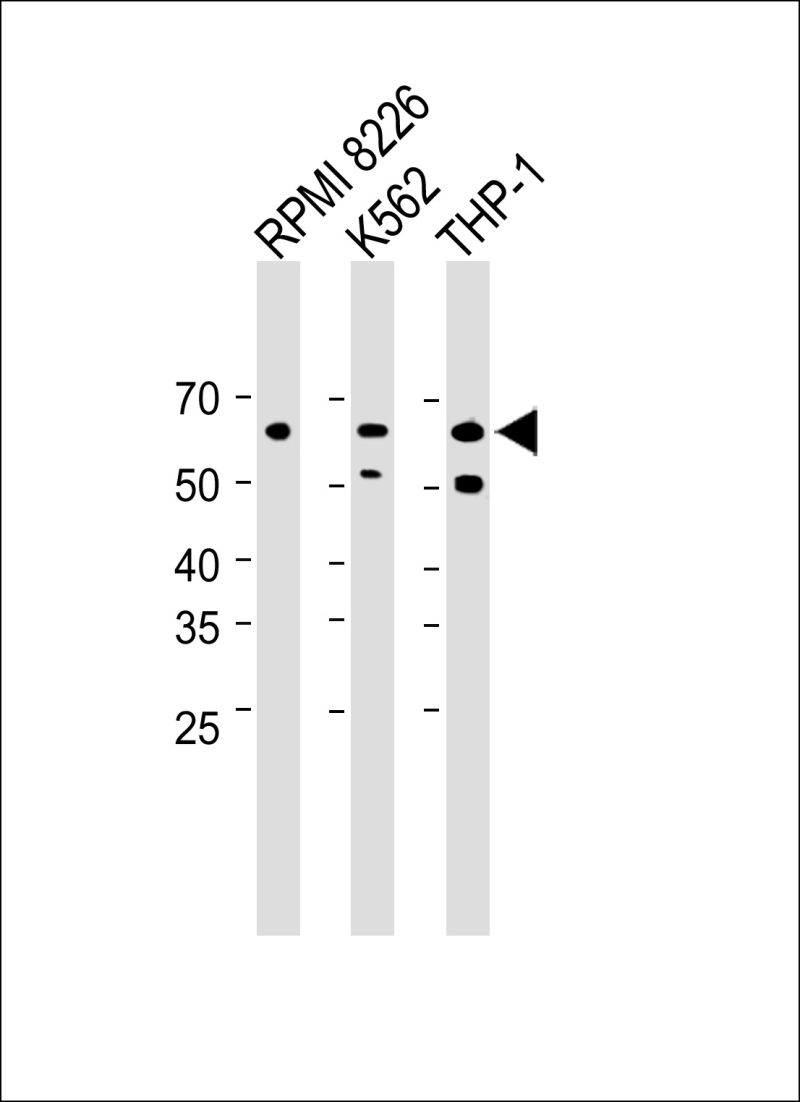
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Trichoplein keratin filament-binding protein, Protein TCHP, Mitochondrial protein with oncostatic activity, Mitostatin, Tumor suppressor protein, TCHP {ECO:0000312|EMBL:AAH042851} |
| Entrez GeneID | 84260 |
| WB Predicted band size | 61.1kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | This TCHP antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 291-320 amino acids from the Central region of human TCHP. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于TCHP方案中抗体(曲妥珠单抗、帕妥珠单抗)的3篇参考文献概览:
---
1. **文献名称**:*Trastuzumab and Pertuzumab in Combination with Carboplatin and Docetaxel for HER2-Positive Breast Cancer*
**作者**:Rimawi MF, et al.
**摘要**:该研究评估TCHP方案(多西他赛、卡铂、曲妥珠单抗、帕妥珠单抗)在HER2阳性乳腺癌新辅助治疗中的疗效。结果显示病理完全缓解率(pCR)达60%,且耐受性良好,支持该方案作为标准治疗方案。
2. **文献名称**:*Cardiac Safety of Dual HER2 Blockade in the TCHP Regimen*
**作者**:Swain SM, et al.
**摘要**:聚焦TCHP方案中曲妥珠单抗和帕妥珠单抗的心脏安全性。长期随访表明,左心室射血分数下降发生率较低(<5%),证实双靶向治疗在严密监测下心脏风险可控。
3. **文献名称**:*Long-term Outcomes of Neoadjuvant TCHP in HER2+ Breast Cancer*
**作者**:Loibl S, et al.
**摘要**:多中心研究分析TCHP新辅助治疗后患者的5年生存率。结果显示无事件生存率(EFS)达85%,强调抗体联合化疗对改善预后的重要性。
---
**备注**:TCHP中的抗体(曲妥珠单抗、帕妥珠单抗)靶向HER2蛋白,通过阻断信号通路抑制肿瘤生长,上述研究聚焦疗效、安全性及长期预后。实际引用时建议通过PubMed或专业数据库核对最新文献。
TCHP antibodies refer to therapeutic agents targeting HER2-positive cancers, primarily breast cancer, within the TCHP regimen—a combination therapy comprising **Trastuzumab** (anti-HER2 monoclonal antibody), **Carboplatin** (chemotherapy), **Docetaxel** (chemotherapy), and **Pertuzumab** (anti-HER2 monoclonal antibody). Trastuzumab, first approved in 1998. binds HER2 receptors to inhibit signaling pathways driving tumor growth. Pertuzumab, approved later (2012), complements this by blocking HER2 dimerization with other HER receptors, enhancing antitumor effects. Both antibodies are humanized IgG1 monoclonal antibodies designed to target distinct HER2 epitopes, leveraging immune-mediated mechanisms (e.g., ADCC) alongside direct signaling blockade.
The TCHP regimen emerged from clinical trials demonstrating superior efficacy in HER2-positive metastatic and early-stage breast cancer, particularly in neoadjuvant settings. The combination’s success lies in dual HER2 inhibition (Trastuzumab + Pertuzumab) synergizing with chemotherapy to reduce tumor burden and improve survival. While effective, these antibodies carry risks like cardiotoxicity and infusion reactions, necessitating careful monitoring. Their development marked a paradigm shift in precision oncology, emphasizing molecular targeting in solid tumors. Ongoing research explores expanding their use and optimizing combination strategies.
×