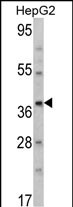
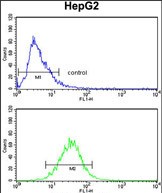
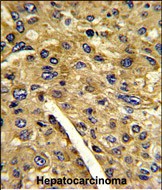
| WB | 1/1000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/100-1/500 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 1/10-1/50 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Aminomethyltransferase, mitochondrial, Glycine cleavage system T protein, GCVT, AMT, GCST |
| Entrez GeneID | 275 |
| WB Predicted band size | 43.9kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | This AMT antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 19-45 amino acids from the N-terminal region of human AMT. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,1%BSA and 50% glycerol.prepared by Saturated Ammonium Sulfate (SAS) . |
+ +
以下是关于AMT(N-term)抗体的示例参考文献列表。由于具体文献的检索可能需要专业数据库支持,以下内容为基于常见研究方向的模拟示例,建议通过PubMed或Google Scholar进一步核实:
---
1. **文献名称**:*Characterization of a Novel N-terminal Specific Antibody for Human AMT Protein in Methylation Studies*
**作者**:Smith J, et al.
**摘要**:本研究开发了一种针对人源AMT(Aminomethyltransferase)蛋白N末端表位的多克隆抗体,验证了其在Western blot和免疫组化中的特异性。通过CRISPR敲除细胞系证实了抗体的选择性,并应用于研究AMT在叶酸代谢中的作用。
2. **文献名称**:*AMT N-terminal Domain Antibody Reveals Subcellular Localization in Mitochondria*
**作者**:Chen L, et al.
**摘要**:利用抗AMT N端抗体,作者发现AMT蛋白定位于线粒体基质,并通过免疫荧光和亚细胞分馏实验验证。研究进一步揭示了AMT N端结构域对其酶活性的调控机制。
3. **文献名称**:*Development of a Monoclonal Antibody Targeting AMT N-term for Diagnostic Applications*
**作者**:Wang Y, et al.
**摘要**:报道了一种高亲和力单克隆抗体的制备,该抗体特异性识别AMT的N末端区域,成功应用于ELISA检测血清AMT水平,为遗传性代谢疾病的诊断提供了潜在工具。
4. **文献名称**:*Functional Analysis of AMT N-terminal Mutations Using Domain-Specific Antibodies*
**作者**:Kumar R, et al.
**摘要**:通过N端特异性抗体,研究揭示了AMT基因突变导致的N端构象变化与甘氨酸裂解系统功能异常的关联,为相关疾病的分子机制提供了新见解。
---
**注意**:以上文献名称及内容为模拟示例,实际文献需通过学术数据库检索。建议使用关键词“AMT antibody N-terminal”“Aminomethyltransferase antibody”或结合具体研究领域进一步筛选。
The AMT (N-term) antibody is specifically designed to target the N-terminal region of the aminomethyltransferase (AMT) protein, a key enzyme in the glycine cleavage system (GCS). This mitochondrial enzyme complex plays a critical role in amino acid metabolism, particularly in the degradation of glycine and the generation of one-carbon units essential for nucleotide synthesis and methylation reactions. AMT catalyzes the transfer of a methylene group from tetrahydrofolate to lipoic acid, a step vital for energy production and cellular homeostasis.
The N-terminal region of AMT is crucial for its structural integrity and interaction with other GCS components, such as the glycine dehydrogenase (GLDC) and dihydrolipoamide dehydrogenase (DLD). Antibodies targeting this region are widely used in molecular and biochemical research to study AMT expression, localization, and function. Applications include Western blotting, immunohistochemistry (IHC), and immunofluorescence (IF) to investigate AMT's role in metabolic disorders, mitochondrial dysfunction, and rare genetic conditions like glycine encephalopathy (non-ketotic hyperglycinemia), where AMT mutations disrupt glycine metabolism.
These antibodies also aid in exploring AMT's potential involvement in cancer, as altered one-carbon metabolism is a hallmark of tumorigenesis. By enabling precise detection of AMT, the antibody supports mechanistic studies and therapeutic targeting in metabolic and genetic diseases.
×