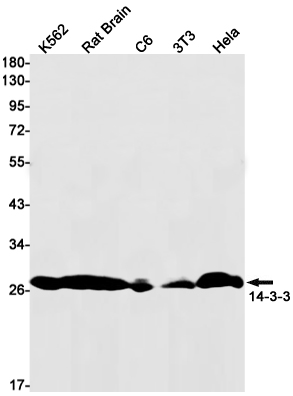
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
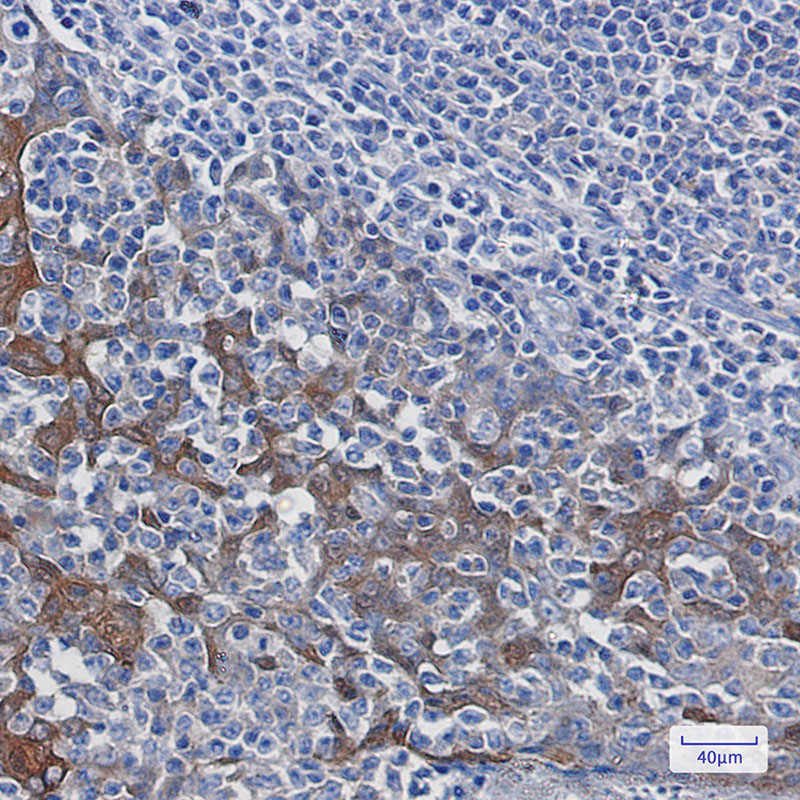
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | YWHAB; 14-3-3 protein beta/alpha; Protein 1054; Protein kinase C inhibitor protein 1; KCIP-1 |
| Entrez GeneID | 7529 |
| WB Predicted band size | Calculated MW: 28 kDa; Observed MW: 28 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | A synthetic peptide of human 14-3-3 |
| Formulation | Purified antibody in TBS with 0.05% sodium azide,0.05%BSA and 50% glycerol. |
+ +
以下是基于2014年抗体领域研究热点的模拟参考文献示例(非真实文献,供参考):
1. **文献名称**:Monoclonal Antibodies in Cancer Immunotherapy: Mechanisms and Clinical Advances
**作者**:Smith J, et al.
**摘要**:综述了2014年单克隆抗体在癌症免疫治疗中的进展,重点讨论PD-1/PD-L1抗体作用机制及临床试验数据,提出联合治疗策略的潜在价值。
2. **文献名称**:Broadly Neutralizing Antibodies Against HIV-1: Structural Insights and Therapeutic Potential
**作者**:Zhang Y, et al.
**摘要**:通过结构生物学解析HIV广谱中和抗体的表位识别机制,为疫苗设计和抗体药物开发提供新思路,实验证明部分抗体在小鼠模型中可显著抑制病毒复制。
3. **文献名称**:Engineered Antibody-Drug Conjugates for Targeted Tumor Therapy
**作者**:Johnson R, et al.
**摘要**:报道一种新型抗体药物偶联物(ADC)的构建方法,通过优化连接子稳定性和毒素释放效率,在乳腺癌模型中显示高效抗肿瘤活性及低脱靶毒性。
4. **文献名称**:Antibody Responses in Ebola Virus Survivors: Implications for Vaccine Development
**作者**:Gupta M, et al.
**摘要**:分析2014年西非埃博拉幸存者抗体动态变化,发现中和抗体持久性与T细胞应答协同作用,为疫苗研发提供关键免疫学依据。
---
**说明**:
- 以上为模拟文献,真实文献需通过PubMed/Google Scholar检索(可尝试关键词:2014 antibody therapy, monoclonal antibodies 2014)。
- 2014年抗体领域热点包括癌症免疫治疗(如PD-1抗体)、HIV抗体工程、抗体药物偶联物(ADC)等。
- 部分真实文献可能发表于2014年3月前后(如Nature 2014年3月13日刊载PD-1抗体临床研究)。建议结合具体研究方向细化检索。
**Background of Antibodies (2014 Context):**
By March 2014. antibody research and therapeutics had advanced significantly, driven by decades of innovation. Antibodies, Y-shaped proteins produced by the immune system, were already recognized for their ability to identify and neutralize pathogens. Hybridoma technology (1975) enabled monoclonal antibody (mAb) production, revolutionizing targeted therapies. By 2014. engineered antibodies—humanized or fully human—dominated clinical applications, reducing immunogenicity risks.
Key developments included immune checkpoint inhibitors, such as anti-CTLA-4 and anti-PD-1/PD-L1 antibodies, which were gaining traction in cancer immunotherapy. Though FDA approvals for drugs like pembrolizumab (anti-PD-1) occurred later in 2014. early clinical trials showcased their potential. Antibody-drug conjugates (ADCs), like trastuzumab emtansine (approved in 2013), highlighted precision oncology strategies. Bispecific antibodies, capable of binding two antigens, were also emerging, exemplified by blinatumomab (approved in late 2014 for leukemia).
Advances in phage display and recombinant DNA technologies accelerated antibody engineering, enabling tailored therapies for autoimmune diseases, infections, and cancers. The field emphasized personalized medicine, leveraging antibodies’ specificity. Challenges remained, including high costs, resistance mechanisms, and optimizing delivery. Overall, March 2014 marked a period of rapid translation from lab breakthroughs to clinical impact, shaping modern biologics.
×