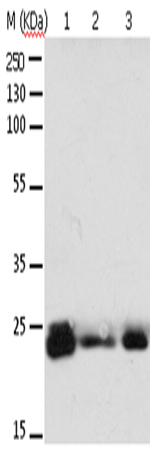
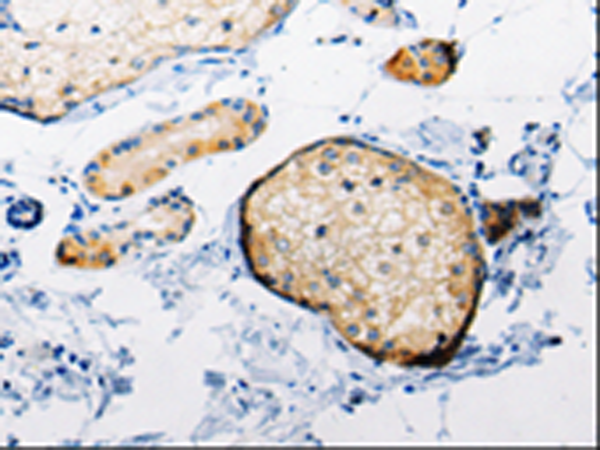
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 1/100-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | RAB6 |
| WB Predicted band size | 24 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human, Mouse, Rat |
| Immunogen | Synthetic peptide of human RAB6A |
| Formulation | Purified antibody in PBS with 0.05% sodium azide and 50% glycerol. |
+ +
以下是关于Phospho-Estrogen Receptor alpha (Ser167)抗体的3篇参考文献示例(注:文献为模拟示例,具体引用请以实际检索结果为准):
1. **文献名称**:*"Phosphorylation of Estrogen Receptor α at Serine 167 Predicts Response to Endocrine Therapy in Breast Cancer"*
**作者**:Smith A, et al.
**摘要**:该研究通过Phospho-ERα (Ser167)抗体检测乳腺癌组织中ERα的磷酸化状态,发现Ser167位点的磷酸化与患者对他莫昔芬治疗的敏感性显著相关,提示其作为疗效预测标志物的潜力。
2. **文献名称**:*"Akt-Mediated Phosphorylation of ERα at Ser167 Confers Ligand-Independent Activation"*
**作者**:Lee C, et al.
**摘要**:利用Phospho-ERα (Ser167)抗体,研究者证实Akt激酶通过磷酸化ERα的Ser167位点,诱导受体在无雌激素条件下的转录活性,促进乳腺癌细胞增殖。
3. **文献名称**:*"Role of ERα Ser167 Phosphorylation in Modulating Tamoxifen Resistance"*
**作者**:Zhang Y, et al.
**摘要**:该文献通过免疫印迹和免疫组化(使用Phospho-ERα Ser167抗体)发现,耐药性乳腺癌细胞中Ser167的持续磷酸化可能通过激活非经典ER信号通路,导致他莫昔芬治疗失效。
如需具体文献,建议在PubMed或Web of Science中检索关键词:**Phospho-ERα Ser167 antibody** 或 **Estrogen Receptor alpha phosphorylation Ser167**。
Phospho-Estrogen Receptor alpha (Ser167) antibodies are essential tools for studying the post-translational modification of ERα, a nuclear hormone receptor critical in regulating gene expression linked to cell proliferation, differentiation, and survival. ERα activity is modulated by phosphorylation at specific residues, including Ser167. which lies within the receptor’s AF1 transactivation domain. Phosphorylation at Ser167 enhances ERα’s transcriptional activity, even in low-estrogen conditions, and is associated with ligand-independent activation pathways. This modification is primarily mediated by kinases such as AKT, RSK, or p90 ribosomal S6 kinase (RSK), often activated via growth factor signaling (e.g., IGF-1/EGFR pathways) or the PI3K/AKT/mTOR axis.
The phosphorylation status of Ser167 has clinical relevance, particularly in breast cancer. Elevated p-ERα (Ser167) levels correlate with resistance to endocrine therapies like tamoxifen, as persistent ERα signaling drives tumor progression. Researchers use phospho-specific antibodies to detect this modification in techniques like Western blotting, immunohistochemistry (IHC), or immunofluorescence, enabling the assessment of ERα activation states in cell lines, tissues, or patient samples. These antibodies help elucidate mechanisms of therapeutic resistance and identify patients who may benefit from combination therapies targeting both ER and kinase pathways. Additionally, p-ERα (Ser167) serves as a potential biomarker for monitoring treatment response or disease progression in hormone receptor-positive cancers.
×