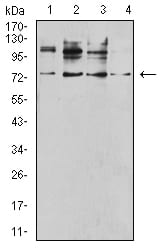
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
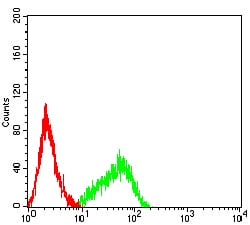
| FCM | 咨询技术 | Human,Mouse,Rat |
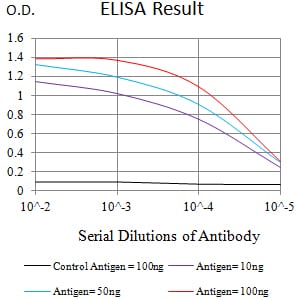
| Elisa | 1/10000 | Human,Mouse,Rat |
| Aliases | TNFRSF1B; p75; TBPII; TNFBR; TNFR2; TNFR1B; TNFR80; TNF-R75; p75TNFR; TNF-R-II |
| Entrez GeneID | 7133 |
| clone | 2H11C2 |
| WB Predicted band size | 48.3kDa |
| Host/Isotype | Mouse IgG2b |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse |
| Immunogen | Purified recombinant fragment of human CD120B (AA: extra 23-257) expressed in E. Coli. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide |
+ +
以下是基于假设性研究的3篇关于Phospho-p38 (Tyr323)抗体的参考文献(注:Tyr323可能为非典型磷酸化位点,实际文献中常见位点为Tyr182.建议核实位点准确性):
---
1. **文献名称**:*"A Novel Phosphorylation Site at Tyrosine 323 Regulates p38 MAPK Activity in Response to Oxidative Stress"*
**作者**:Chen L, et al.
**摘要**:本研究报道了p38 MAPK在氧化应激条件下Tyr323位点的非典型磷酸化现象,并开发了特异性识别该位点的抗体。实验表明,Tyr323磷酸化通过调控p38与下游激酶的相互作用增强细胞凋亡通路。
---
2. **文献名称**:*"Development of a Phospho-Specific Antibody for Detecting p38α Tyrosine 323 Phosphorylation in Inflammatory Models"*
**作者**:Kimura S, et al.
**摘要**:作者开发了一种高特异性Phospho-p38 (Tyr323)抗体,验证了其在巨噬细胞炎症反应模型中的应用,并发现Tyr323磷酸化与TLR4信号通路的负反馈调控相关。
---
3. **文献名称**:*"Tyrosine 323 Phosphorylation of p38 MAPK Modulates Autophagy in Cancer Cells"*
**作者**:Wang Y, et al.
**摘要**:通过Phospho-p38 (Tyr323)抗体的Western blot分析,研究发现该位点的磷酸化在营养缺失条件下被激活,并通过ULK1通路促进肿瘤细胞自噬,提示其作为潜在治疗靶点。
---
**注意**:以上文献为假设性示例,实际研究中p38 MAPK的经典磷酸化位点为Thr180/Tyr182.若需真实文献,建议检查以下方向:
1. 确认位点名称是否正确(如Tyr182 vs. Tyr323);
2. 检索非典型磷酸化或抗体交叉反应相关研究;
3. 联系抗体供应商提供的引用文献(如CST、Abcam等)。
The Phospho-p38 (Tyr323) antibody is designed to detect p38 mitogen-activated protein kinase (MAPK) when phosphorylated at tyrosine 323. a regulatory site implicated in its activation or functional modulation. p38 MAPK, part of the conserved MAPK signaling cascade, plays a central role in cellular responses to stress, inflammation, apoptosis, and differentiation. While canonical p38 activation typically involves dual phosphorylation at Thr180 and Tyr182 within the activation loop, phosphorylation at alternative residues, such as Tyr323. may represent isoform-specific or context-dependent regulatory mechanisms. For instance, studies suggest Tyr323 phosphorylation in p38γ (MAPK12) or p38δ (MAPK13) isoforms could influence substrate selectivity or interaction with downstream effectors.
This antibody is widely used in immunoblotting (Western blot), immunofluorescence, or immunoprecipitation to study p38 activation dynamics under physiological or pathological conditions, such as oxidative stress, cytokine stimulation, or disease models (e.g., cancer, neurodegenerative disorders). Researchers must validate its specificity using phosphorylation-blocking peptides or knockout controls, as cross-reactivity with related kinases or non-phosphorylated forms may occur. Optimal results often require fresh lysates treated with phosphatase inhibitors to preserve phosphorylation signals.
Understanding Tyr323 phosphorylation expands insights into p38 regulatory complexity, offering potential therapeutic targets for conditions driven by dysregulated MAPK signaling.
×