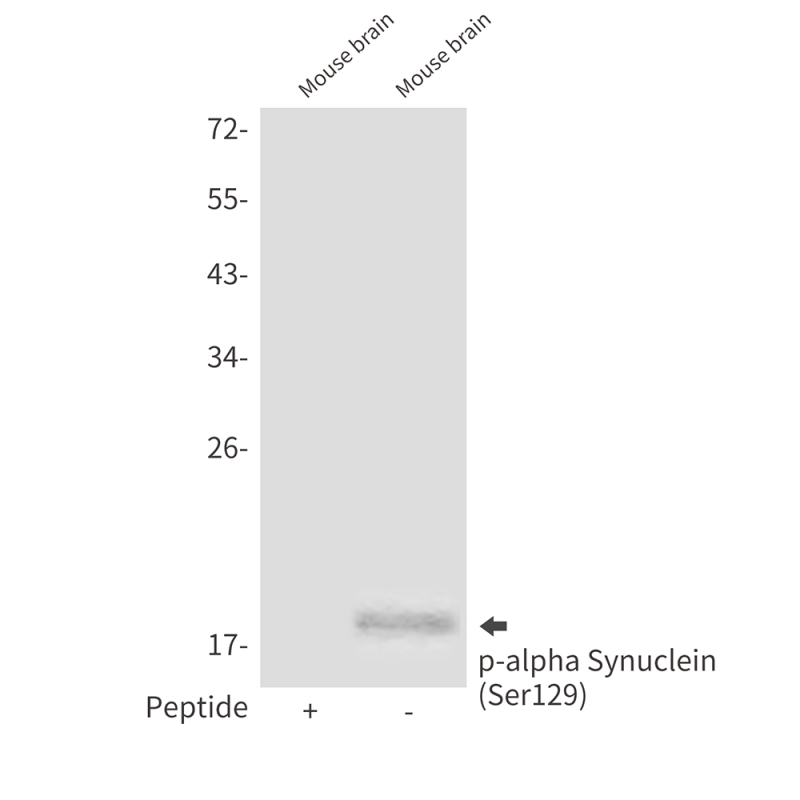
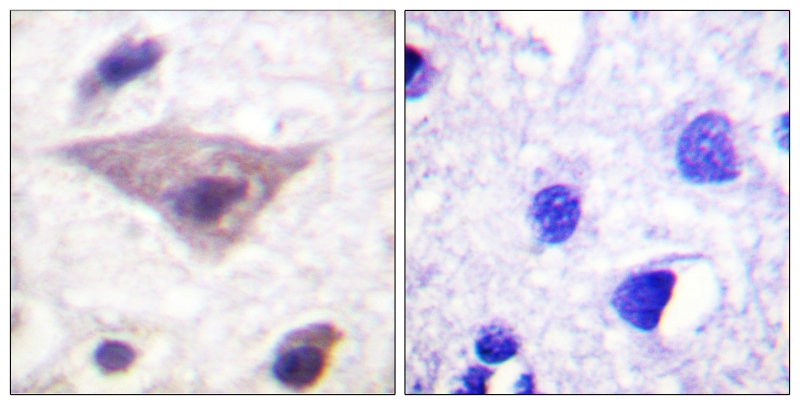
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | SNCA; NACP; PARK1; Alpha-synuclein; Non-A beta component of AD amyloid; Non-A4 component of amyloid precursor; NACP |
| Entrez GeneID | 6622 |
| WB Predicted band size | Calculated MW: 14 kDa; Observed MW: 18 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Synthetic peptide of human SNCA |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
1. **"Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease"**
- **作者**: Fujiwara H, et al.
- **摘要**: 该研究首次证实α-突触核蛋白在Ser129位点的磷酸化(pS129)是帕金森病和路易体痴呆中路易小体的主要病理标志,通过特异性抗体揭示了其在神经病理中的广泛存在。
2. **"Pathological α-synuclein transmission initiated by binding lymphocyte-activation gene 3 (LAG3)"**
- **作者**: Mao X, et al.
- **摘要**: 研究利用pS129抗体追踪α-突触核蛋白原纤维在细胞和小鼠模型中的传播机制,发现LAG3蛋白介导病理性α-syn的内吞,推动神经退行性病变。
3. **"A novel α-synuclein seed amplification assay for detecting Lewy body pathology in cerebrospinal fluid"**
- **作者**: Shahnawaz M, et al.
- **摘要**: 开发了一种基于pS129抗体检测脑脊液中α-syn病理性聚集物的方法,证明其在帕金森病和路易体痴呆患者中具有高敏感性和特异性,可作为早期诊断标志物。
4. **"Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death"**
- **作者**: Volpicelli-Daley LA, et al.
- **摘要**: 通过pS129抗体证实外源性α-syn原纤维可诱导神经元内路易样包涵体形成,并导致突触功能障碍,为病理传播机制提供了实验依据。
Phospho-alpha Synuclein (Ser129) antibodies are critical tools in studying the pathogenesis of neurodegenerative disorders, particularly Parkinson’s disease (PD) and other synucleinopathies. Alpha-synuclein (α-Syn) is a presynaptic protein implicated in synaptic vesicle regulation. In pathological conditions, it aggregates into insoluble fibrils, forming Lewy bodies and Lewy neurites—hallmark lesions in these diseases. Post-translational modifications, including phosphorylation at serine 129 (Ser129), are linked to α-Syn aggregation. Notably, over 90% of aggregated α-Syn in Lewy bodies is phosphorylated at Ser129. suggesting this modification plays a key role in disease progression.
Phospho-specific antibodies targeting Ser129 enable researchers to detect and quantify this modified form of α-Syn in experimental models and human tissues. These antibodies are widely used in techniques like immunohistochemistry, Western blotting, and ELISA to study phosphorylation dynamics in cellular and animal models of neurodegeneration. Their application has advanced understanding of how Ser129 phosphorylation influences α-Syn toxicity, aggregation, and clearance mechanisms.
Additionally, these antibodies hold diagnostic potential, as elevated phospho-Ser129 α-Syn levels in cerebrospinal fluid or blood are explored as biomarkers for early disease detection. However, debates persist about whether Ser129 phosphorylation promotes aggregation or represents a cellular protective response. Continued research using these antibodies may clarify its role and guide therapeutic strategies targeting α-Syn pathology.
×