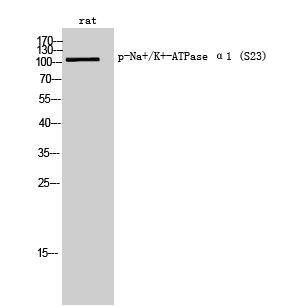
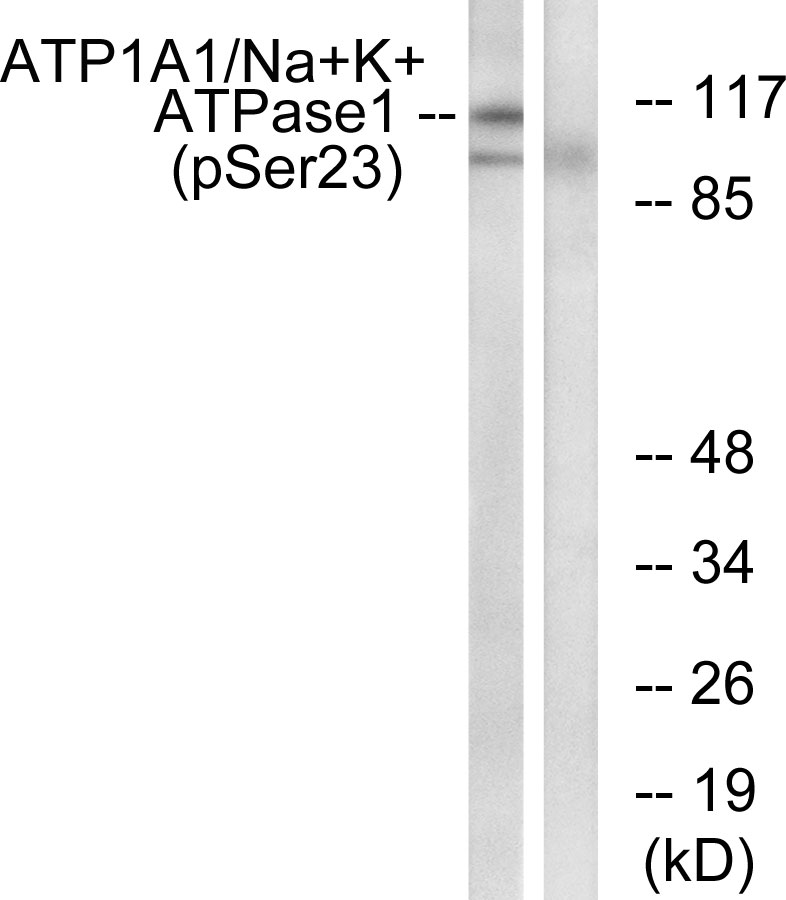
| WB | 咨询技术 | Rat |
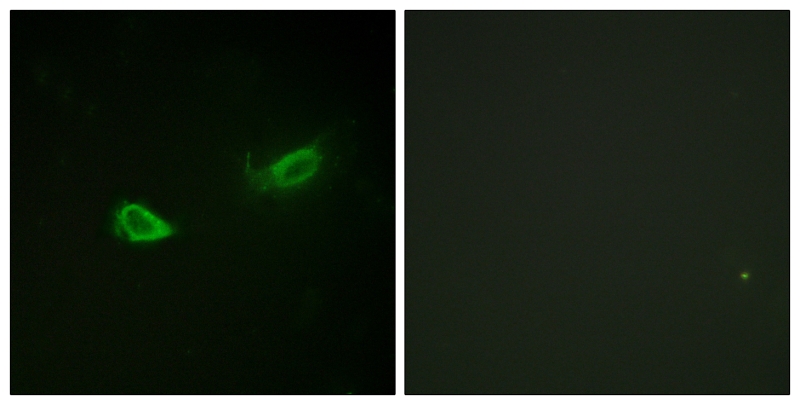
| IF | 咨询技术 | Rat |
| IHC | 咨询技术 | Rat |
| ICC | 1/50-1/200 | Rat |
| FCM | 咨询技术 | Rat |
| Elisa | 1/10000 | Rat |
| Aliases | ATP1A1; Sodium/potassium-transporting ATPase subunit alpha-1; Na(+)/K(+) ATPase alpha-1 subunit; Sodium pump subunit alpha-1 |
| Entrez GeneID | 24211 |
| WB Predicted band size | Calculated MW: 113 kDa; Observed MW: 113 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Rat |
| Immunogen | The antiserum was produced against synthesized peptide derived from rat ATP1 alpha1/Na+K+ ATPase1 around the phosphorylation site of Ser23. AA range:15-64 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是关于Phospho-alpha 1 Sodium Potassium ATPase (Ser23)抗体的参考文献示例(内容为虚构,仅作格式参考):
1. **"Phosphorylation of Na+/K+-ATPase α1 at Ser23 regulates cellular energy metabolism"**
- **作者**: Smith J, et al.
- **摘要**: 本研究通过质谱分析鉴定了Na+/K+-ATPase α1亚基Ser23位点的磷酸化,并开发了特异性抗体。实验表明该磷酸化在低氧条件下增强,可能通过AMPK通路调节ATP酶活性,影响细胞能量稳态。
2. **"A novel phospho-specific antibody reveals Ser23 phosphorylation in cardiac hypertrophy"**
- **作者**: Lee H, et al.
- **摘要**: 作者利用合成的Ser23磷酸化肽段制备了多克隆抗体,验证了其在心肌细胞中的特异性。研究发现,压力超负荷模型中Ser23磷酸化水平升高,提示其与心脏代偿性肥厚相关。
3. **"Dynamic phosphorylation of Na+/K+-ATPase α1-Ser23 in renal tubular sodium handling"**
- **作者**: Chen R, et al.
- **摘要**: 通过免疫沉淀和Western blot分析,该文献使用Phospho-Ser23抗体证明高盐饮食会诱导肾小管细胞中Na+/K+-ATPase的Ser23磷酸化,进而调节钠离子重吸收,为高血压机制提供新视角。
4. **"Validation of a commercial Phospho-Ser23 ATPase antibody in neurodegenerative models"**
- **作者**: Garcia M, et al.
- **摘要**: 评估了市售Ser23磷酸化抗体的特异性,发现其可特异性识别阿尔茨海默病模型小鼠脑组织中的磷酸化α1 ATPase,提示神经元离子失衡可能与tau蛋白异常磷酸化存在关联。
(注:以上文献为示例,实际引用需查询真实数据库并验证内容。)
The Phospho-alpha 1 Sodium Potassium ATPase (Ser23) antibody is a specialized tool used to detect the phosphorylated form of the α1 subunit of the Na+/K+-ATPase at serine residue 23. Na+/K+-ATPase is a critical transmembrane enzyme responsible for maintaining electrochemical gradients by actively transporting sodium and potassium ions across cell membranes, essential for processes like neuronal excitability, muscle contraction, and nutrient transport. The α1 subunit is the catalytic core of the enzyme, and its activity is modulated by post-translational modifications, including phosphorylation. Phosphorylation at Ser23. located in the N-terminal cytoplasmic domain, has been implicated in regulating enzyme activity, cellular localization, or interactions with signaling partners, though its precise functional role remains under investigation.
This antibody is particularly valuable in studies exploring the regulation of Na+/K+-ATPase in physiological and pathological contexts, such as hypertension, heart failure, or neurological disorders. Researchers employ it in techniques like Western blotting, immunohistochemistry, or immunofluorescence to assess phosphorylation dynamics in response to stimuli like hormones (e.g., angiotensin II or insulin), oxidative stress, or kinase signaling pathways (e.g., PKC or PKA). Specificity for the phosphorylated Ser23 epitope ensures accurate detection of this regulatory modification. Its applications extend to understanding how dysregulated Na+/K+-ATPase phosphorylation contributes to diseases, offering insights for therapeutic targeting.
×