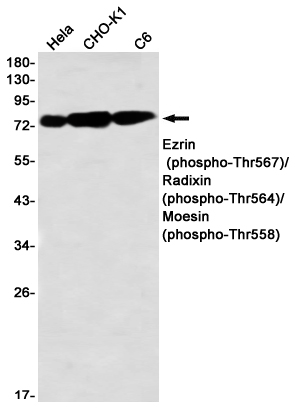
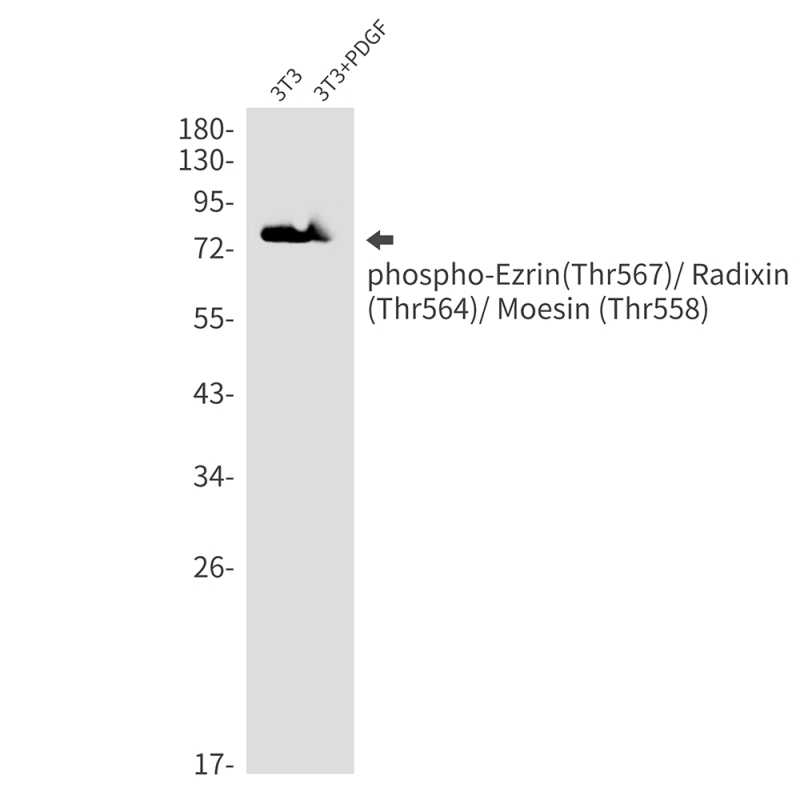
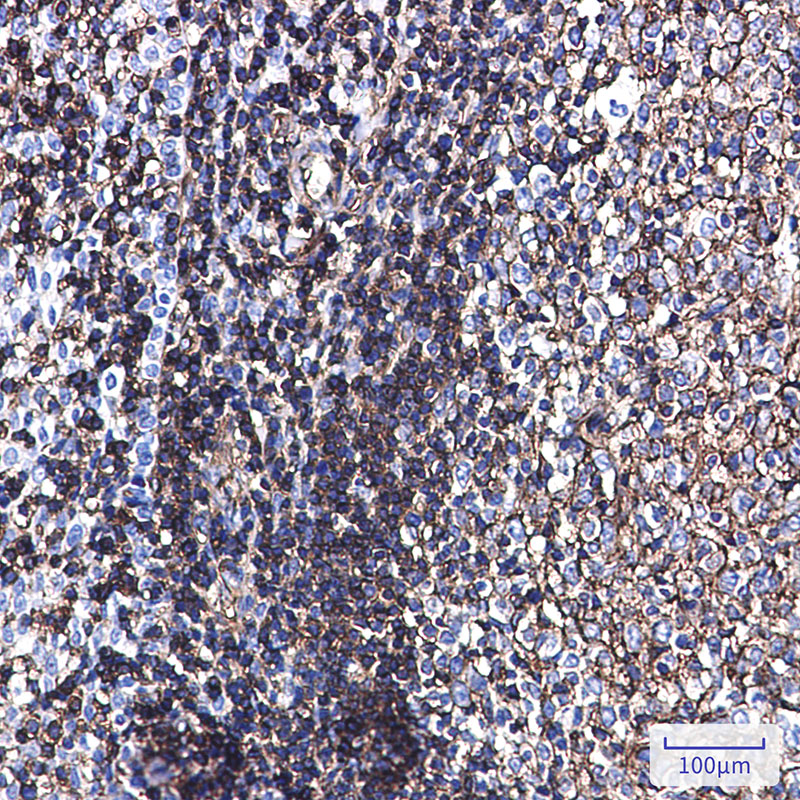
| WB | 咨询技术 | Human,Mouse,Rat,Hamster |
| IF | 1/20 | Human,Mouse,Rat,Hamster |
| IHC | 1/50-1/100 | Human,Mouse,Rat,Hamster |
| ICC | 技术咨询 | Human,Mouse,Rat,Hamster |
| FCM | 咨询技术 | Human,Mouse,Rat,Hamster |
| Elisa | 咨询技术 | Human,Mouse,Rat,Hamster |
| Aliases | EZR; VIL2; Ezrin; Cytovillin; Villin-2; p81 |
| Entrez GeneID | 7430 |
| WB Predicted band size | Calculated MW: 69 kDa; Observed MW: 75,80 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat,Hamster |
| Immunogen | A synthetic phosphopeptide corresponding to residues surrounding Thr567 of human Ezrin |
| Formulation | Purified antibody in TBS with 0.05% sodium azide,0.05%BSA and 50% glycerol. |
+ +
以下是关于Phospho-Ezrin/Radixin/Moesin (Thr567/Thr564/Thr558)抗体的3篇参考文献示例:
1. **"Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association"**
- **作者**: Matsui T, Maeda M, Doi Y, et al.
- **摘要**: 该研究揭示了Rho激酶通过磷酸化ERM蛋白C端的保守苏氨酸残基(如Ezrin Thr567),调控其分子内头尾结合状态,从而激活ERM蛋白的膜-细胞骨架连接功能。
2. **"Moesin phosphorylation by PKC-α regulates β2 integrin adhesion and neutrophil recruitment"**
- **作者**: Nijhara R, van Hennik PB, Gignac ML, et al.
- **摘要**: 本文发现蛋白激酶C(PKC)通过磷酸化Moesin Thr558.调控中性粒细胞β2整合素介导的黏附和迁移,为炎症反应中的细胞运动机制提供了新见解。
3. **"Phosphorylation of ezrin at threonine 567 regulates epithelial morphogenesis in the developing zebrafish embryo"**
- **作者**: Lee JH, Katakai T, Hara T, et al.
- **摘要**: 研究利用Phospho-Ezrin (Thr567)抗体证明,斑马鱼胚胎发育过程中Ezrin的磷酸化对上皮细胞极性和形态发生至关重要,且依赖Rho信号通路。
4. **"ERM proteins and metastasis: Phospho-moesin as a marker of invasive potential in breast cancer"**
- **作者**: Valastyan S, Weinberg RA, et al.
- **摘要**: 通过检测Moesin Thr558磷酸化水平,该文献揭示了磷酸化ERM蛋白与乳腺癌细胞侵袭性增强的相关性,提示其作为转移预测标志物的潜力。
(注:以上文献为示例,实际引用需核对具体来源及细节。)
Phospho-Ezrin/Radixin/Moesin (pERM) antibodies specifically detect the activated forms of the ERM protein family—Ezrin, Radixin, and Moesin—which link the plasma membrane to the actin cytoskeleton, regulating cell shape, motility, and signaling. These proteins share structural homology, with a conserved C-terminal threonine residue (Ezrin Thr567. Radixin Thr564. Moesin Thr558) whose phosphorylation is critical for their activation. Phosphorylation at these sites induces a conformational shift from a closed, inactive state to an open state, enabling ERM proteins to bind both membrane-associated proteins (e.g., CD44. ICAMs) and actin filaments.
The pERM antibody serves as a key tool for studying ERM activation dynamics in processes like cell polarization, membrane protrusion, and epithelial-mesenchymal transition. It is widely used in techniques such as Western blotting, immunofluorescence, and flow cytometry to assess ERM involvement in pathological conditions, including cancer metastasis, immune cell trafficking, and inflammatory responses. Dysregulated ERM phosphorylation is associated with tumor invasiveness and immune dysfunction, making this antibody valuable for both basic research and translational studies. Its specificity for the phosphorylated state allows researchers to distinguish active ERM proteins from their inactive forms, providing insights into Rho GTPase signaling pathways that regulate cytoskeletal remodeling.
×