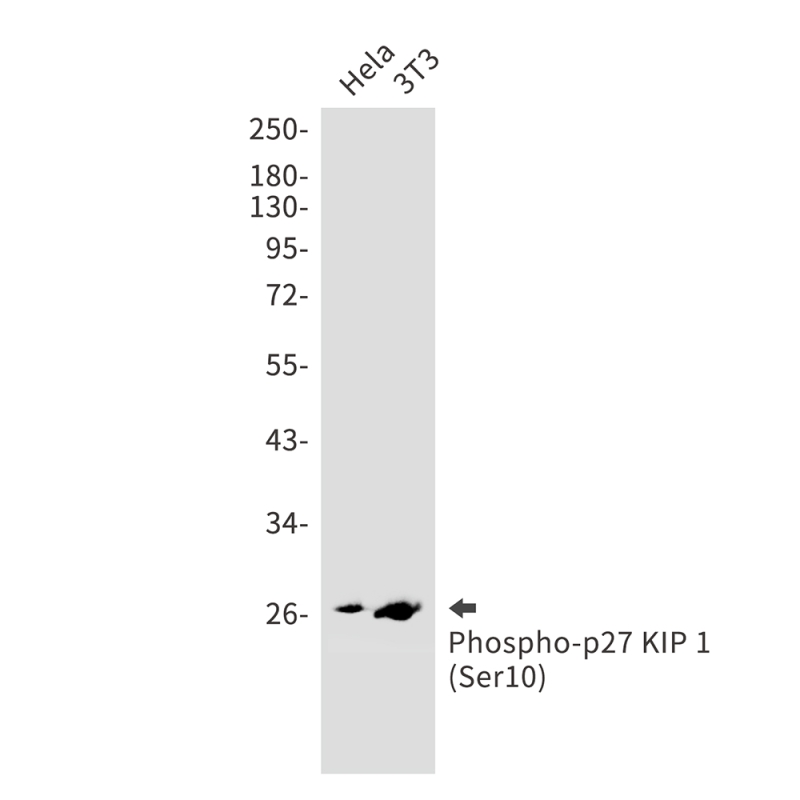
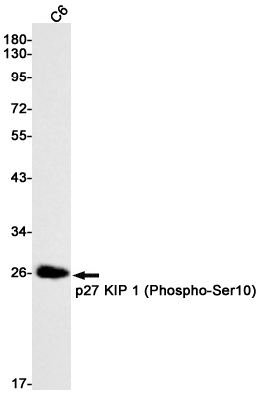
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 1/20 | Human,Mouse,Rat |
| IHC | 1/50-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | CDKN1B; KIP1; Cyclin-dependent kinase inhibitor 1B; Cyclin-dependent kinase inhibitor p27; p27Kip1 |
| Entrez GeneID | 1027 |
| WB Predicted band size | Calculated MW: 22 kDa; Observed MW: 27 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | A synthetic phosphopeptide corresponding to residues surrounding Ser10 of human p27 KIP 1 |
| Formulation | Purified antibody in TBS with 0.05% sodium azide,0.05%BSA and 50% glycerol. |
+ +
以下是3篇关于Phospho-p27 Kip1 (Ser10)抗体的参考文献,按研究内容分类整理:
---
1. **文献名称**:*p27 phosphorylation by Src regulates inhibition of cyclin E-Cdk2*
**作者**:Tsvetkov, L.M. et al.
**摘要**:该研究首次报道了c-Src激酶介导p27在Ser10位点的磷酸化,揭示了该修饰通过促进p27的核输出并增强其蛋白酶体依赖性降解,从而调控细胞周期进程。实验中使用Phospho-Ser10抗体验证了该位点的磷酸化与p27稳定性之间的关系。
---
2. **文献名称**:*Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb binding as Cdk activity rises during G1 progression*
**作者**:Rodier, A. et al.
**摘要**:研究探讨p27在G1期不同阶段的磷酸化动态,发现Ser10位点的磷酸化(通过Phospho-p27抗体检测)由KIS激酶催化,导致p27从核转移到胞质,解除其对CDK2的抑制,促进细胞周期进入S期。
---
3. **文献名称**:*A role for p27/Kip1 in the control of cerebellar granule cell precursor proliferation*
**作者**:Tomoda, H. et al.
**摘要**:利用Phospho-Ser10特异性抗体,作者证明小脑颗粒细胞前体增殖过程中,p27的Ser10磷酸化水平与细胞质定位呈正相关,提示该修饰在神经发育中通过调节p27亚细胞分布影响细胞周期退出。
---
**可选补充**:
4. **文献名称**:*Stability regulation of the CDK inhibitor p27 by phosphorylation and ubiquitination*
**作者**:Besson, A. et al.
**摘要**:该综述系统总结了p27的多个磷酸化位点(包括Ser10)对其蛋白稳定性及功能的调控机制,特别指出Phospho-Ser10抗体在区分不同功能状态p27中的应用价值。
---
这些文献涵盖了该抗体在机制研究(磷酸化与降解)、细胞周期模型验证及组织特异性研究中的关键应用。
The Phospho-p27 Kip1 (Ser10) antibody detects p27 Kip1 (also known as CDKN1B) when phosphorylated at serine 10. a post-translational modification critical for regulating its cellular localization and stability. p27 Kip1 is a cyclin-dependent kinase (CDK) inhibitor that controls cell cycle progression by binding to and inhibiting CDK complexes, particularly during the G1 phase. Phosphorylation at Ser10 promotes cytoplasmic translocation of p27. reducing its nuclear levels and attenuating its cell cycle inhibitory function. This modification is often mediated by kinases such as Akt or other signaling pathways activated in response to mitogenic or oncogenic stimuli.
The Ser10-phosphorylated form of p27 is associated with increased cell proliferation, tumor progression, and poor prognosis in certain cancers. Researchers use the Phospho-p27 Kip1 (Ser10) antibody to study mechanisms underlying cell cycle dysregulation, cancer biology, and therapeutic responses in models ranging from cultured cells to clinical tissue samples. Detection of this epitope via techniques like Western blotting, immunofluorescence, or immunohistochemistry helps elucidate how post-translational modifications of p27 influence its degradation (via ubiquitin-proteasome pathways), subcellular distribution, and interaction partners. Understanding Ser10 phosphorylation provides insights into pathological conditions marked by uncontrolled cell growth, such as carcinomas, and potential targets for restoring cell cycle control.
×