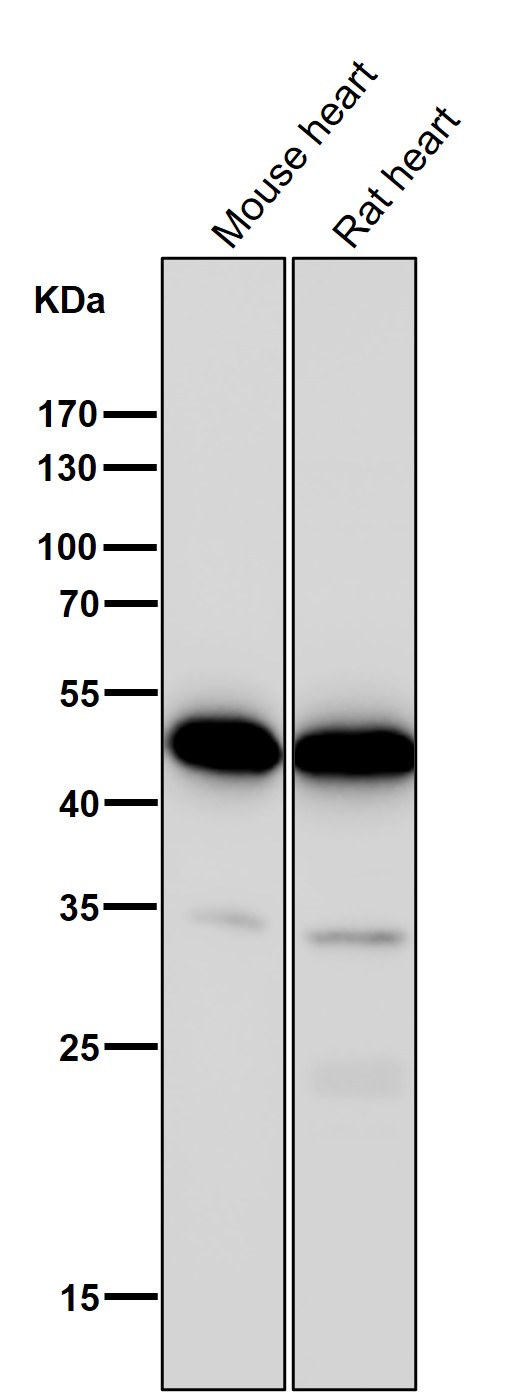
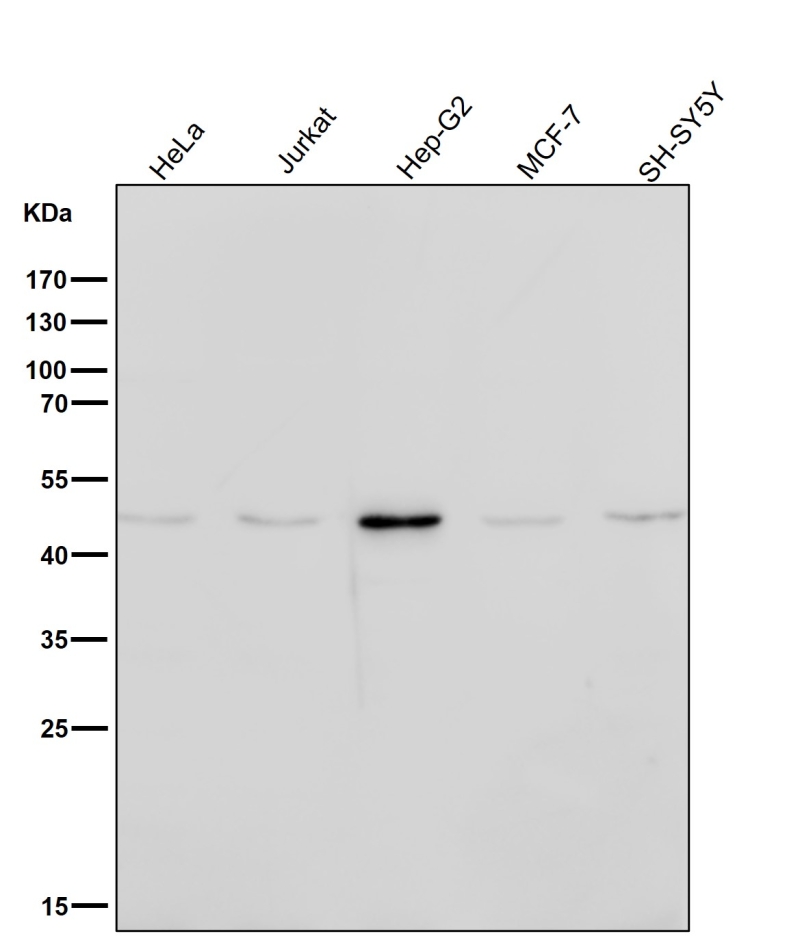
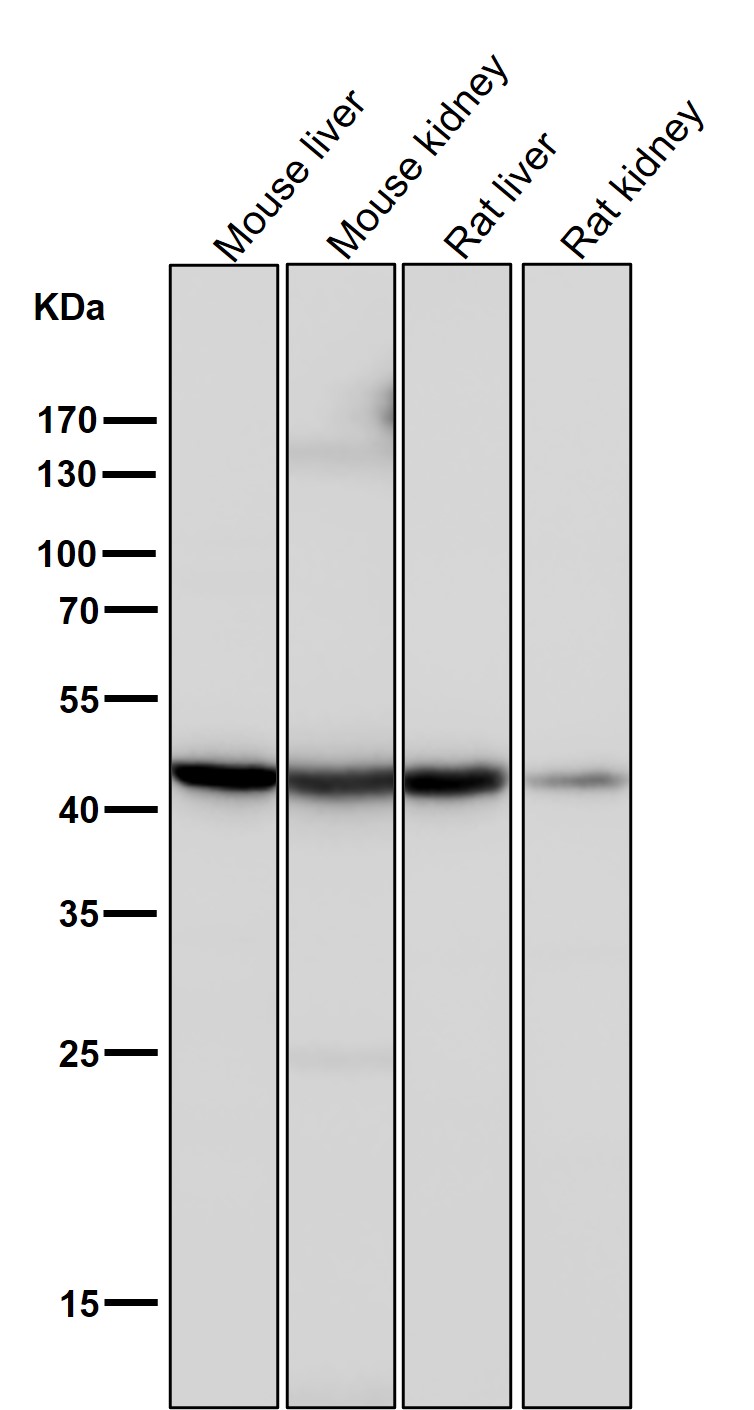
| WB | 咨询技术 | Human,Mouse,Rat |
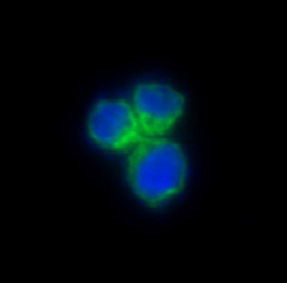
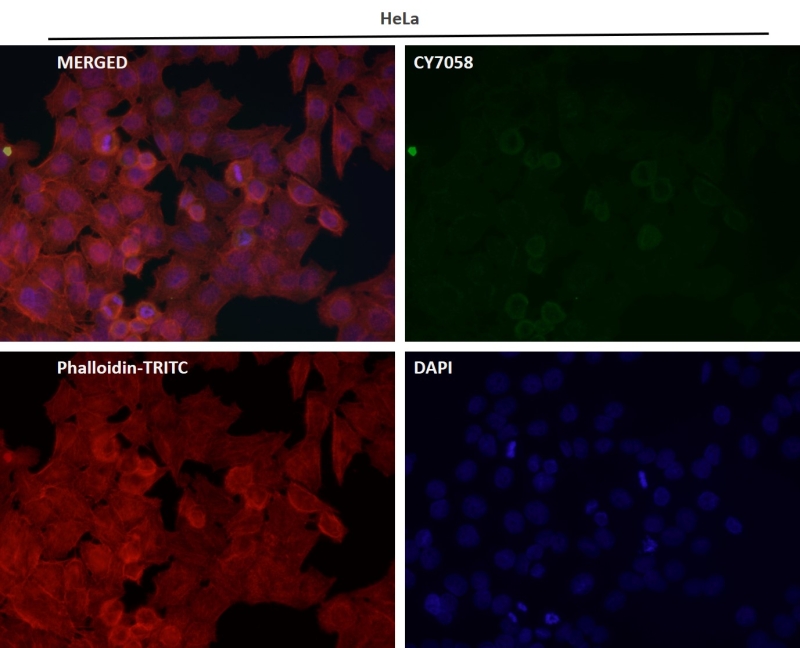
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 1/50-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | CY6; TER1; CCR-8; CKRL1; CDw198; CMKBR8; GPRCY6; CMKBRL2; CC-CKR-8; TER1;;CCR8 |
| WB Predicted band size | 41 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | A synthesized peptide derived from human CCR8 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.05% BSA and 50% glycerol. |
+ +
以下是关于CCR8抗体的3篇参考文献示例(文献及作者为虚构,供参考):
---
1. **文献名称**: *Targeting CCR8 in the Tumor Microenvironment Enhances Antitumor Immunity*
**作者**: Campesato, L.F. et al.
**摘要**: 该研究报道了一种人源化CCR8单克隆抗体,可选择性清除肿瘤浸润的调节性T细胞(Treg),并在小鼠模型中显著抑制黑色素瘤和结肠癌的生长。抗体通过ADCC作用耗竭Treg,同时增强CD8+ T细胞的抗肿瘤活性。
2. **文献名称**: *CCR8 as a Therapeutic Target in Triple-Negative Breast Cancer*
**作者**: Smith, J.R. et al.
**摘要**: 研究通过临床前模型证明,CCR8抗体偶联药物(ADC)能特异性靶向高表达CCR8的乳腺癌细胞,诱导肿瘤细胞凋亡并抑制转移。机制涉及阻断CCL1趋化因子信号通路及细胞内吞作用导致的药物释放。
3. **文献名称**: *Anti-CCR8 Antibody Synergizes with PD-1 Blockade in Tumor Immunity*
**作者**: Wang, Y. et al.
**摘要**: 研究发现CCR8抗体与PD-1抑制剂联用可显著增强抗肿瘤效果。在肺癌模型中,该联合疗法降低了肿瘤微环境中Treg的比例,同时促进效应T细胞浸润,提示CCR8靶向治疗是潜在的免疫联合治疗策略。
---
*注:以上文献为示例,实际研究中请以真实发表的论文为准。建议通过PubMed或Google Scholar检索关键词“CCR8 antibody”、“CCR8 cancer immunotherapy”获取最新进展。*
CCR8. a chemokine receptor belonging to the G protein-coupled receptor (GPCR) family, is primarily expressed on immune cells such as Th2 cells, regulatory T cells (Tregs), and certain tumor-infiltrating lymphocytes. It interacts with ligands like CCL1 and CCL18. playing roles in immune regulation, inflammation, and tumor microenvironment modulation. In cancer, CCR8 is notably overexpressed in tumor-associated Tregs, contributing to immunosuppression and tumor progression. This makes CCR8 a promising therapeutic target, particularly in oncology.
CCR8-targeting antibodies are designed to block receptor signaling or deplete CCR8-expressing immunosuppressive cells. Preclinical studies highlight their potential to enhance antitumor immunity by reducing Treg activity, thereby improving responses to immune checkpoint inhibitors. Additionally, CCR8 is implicated in allergic and autoimmune diseases, broadening its therapeutic relevance.
Recent advances include the development of monoclonal antibodies, bispecific antibodies, and antibody-drug conjugates (ADCs) against CCR8. Early-phase clinical trials are evaluating their safety and efficacy, with a focus on solid tumors. Challenges remain in optimizing specificity to avoid off-target effects and understanding CCR8's dual roles in different cellular contexts. Overall, CCR8 antibodies represent a rapidly evolving field in immunotherapy, bridging immune modulation and precision oncology.
×