
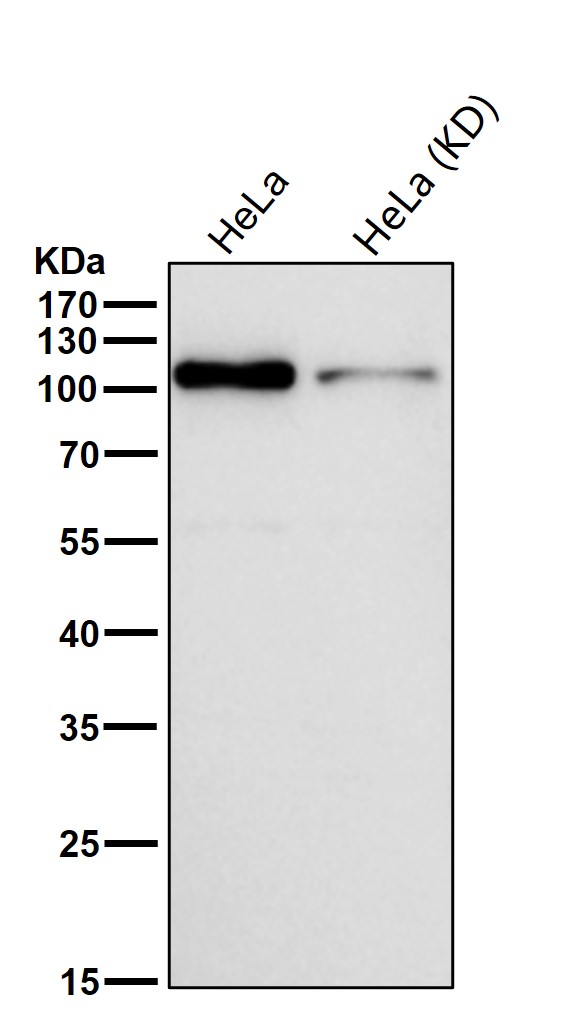
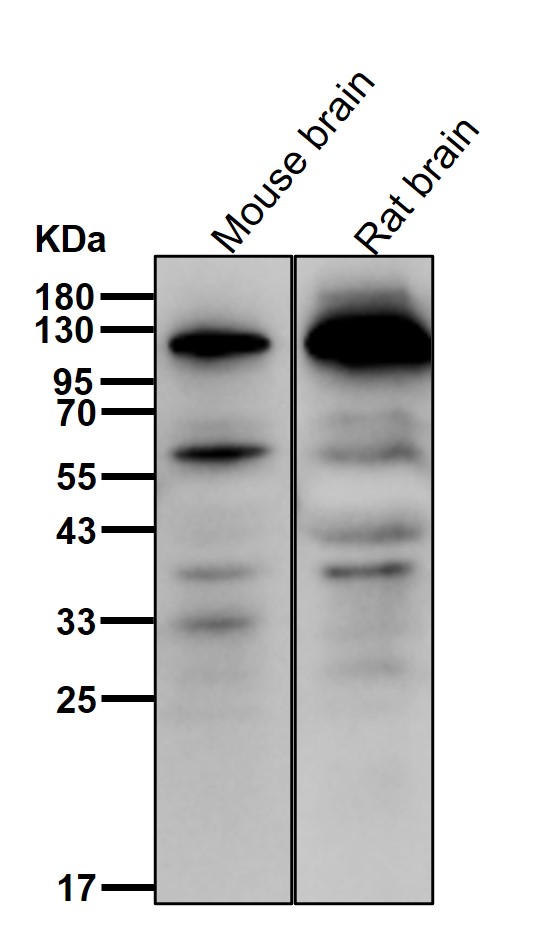
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 1/20-1/50 | Human,Mouse,Rat |
| IHC | IHC:1/100-1/200;IHF:1/50-1/200 | Human,Mouse,Rat |
| ICC | 1/50-1/200 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Antigen NY-CO-25; Heat shock protein 105 kDa; HS105; HSP105; HSP105A; HSP105B; HSP110; HSPH1;;Hsp105 |
| WB Predicted band size | Calculated MW: 97 kDa ; Observed MW: 105 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | A synthesized peptide derived from human Hsp105 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.05% BSA and 50% glycerol. |
+ +
以下是关于Hsp105抗体的3篇代表性文献(信息基于公开研究总结,非实时数据库检索):
1. **文献名称**:*Hsp105α regulates the localization of Alzheimer's disease-associated amyloid precursor protein in stress granules*
**作者**:Hattori, K., et al.
**摘要**:研究利用Hsp105抗体探讨Hsp105α与阿尔茨海默病中淀粉样前体蛋白(APP)的相互作用,发现Hsp105通过调控应激颗粒(stress granules)影响APP的聚集和毒性。
2. **文献名称**:*Heat shock protein 105 is overexpressed in human glioblastoma and regulates cancer stem cell function*
**作者**:Yokota, S., et al.
**摘要**:通过Hsp105抗体检测胶质母细胞瘤组织,发现Hsp105在肿瘤干细胞中高表达,并通过PI3K/Akt通路促进肿瘤增殖和耐药性。
3. **文献名称**:*Hsp105 interacts with Hsp70 and enhances its chaperone activity*
**作者**:Yamagishi, N., et al.
**摘要**:利用Hsp105抗体进行免疫共沉淀实验,证明Hsp105与Hsp70形成复合物,协同增强对错误折叠蛋白的修复能力,尤其在热应激条件下作用显著。
如需具体文献来源,建议通过PubMed或Google Scholar以“Hsp105 antibody”及上述作者名组合检索获取全文信息。
Hsp105 (Heat Shock Protein 105) is a member of the HSP70/HSP110 molecular chaperone family, playing a critical role in cellular stress responses and protein homeostasis. Primarily induced under stress conditions such as heat shock, oxidative stress, or proteotoxic insults, Hsp105 assists in protein folding, prevents aggregation of misfolded proteins, and facilitates their refolding or degradation. Structurally, it contains an N-terminal ATPase domain and a C-terminal substrate-binding domain, enabling its chaperone activity through ATP-dependent conformational changes.
Hsp105 exists in two major isoforms: the stress-inducible HSP105α and the constitutively expressed HSP105β. These isoforms exhibit distinct regulatory roles, with HSP105α predominantly activated during stress to enhance cell survival, while HSP105β contributes to basal protein quality control. Dysregulation of Hsp105 has been implicated in neurodegenerative diseases (e.g., Alzheimer's, Huntington's) due to its interaction with pathogenic protein aggregates, as well as in cancer, where it may promote tumor progression by supporting cancer cell survival under microenvironmental stress.
Antibodies targeting Hsp105 are essential tools for investigating its expression, localization, and function. They are widely used in techniques like Western blotting, immunohistochemistry, and immunofluorescence to study stress response mechanisms, disease pathology, and therapeutic targeting. Commercially available antibodies often distinguish between isoforms or post-translationally modified forms, enabling precise research into Hsp105's dual roles as a cytoprotective agent and potential disease driver. Recent studies also explore its diagnostic potential as a biomarker in cancers and neurodegenerative disorders.
×