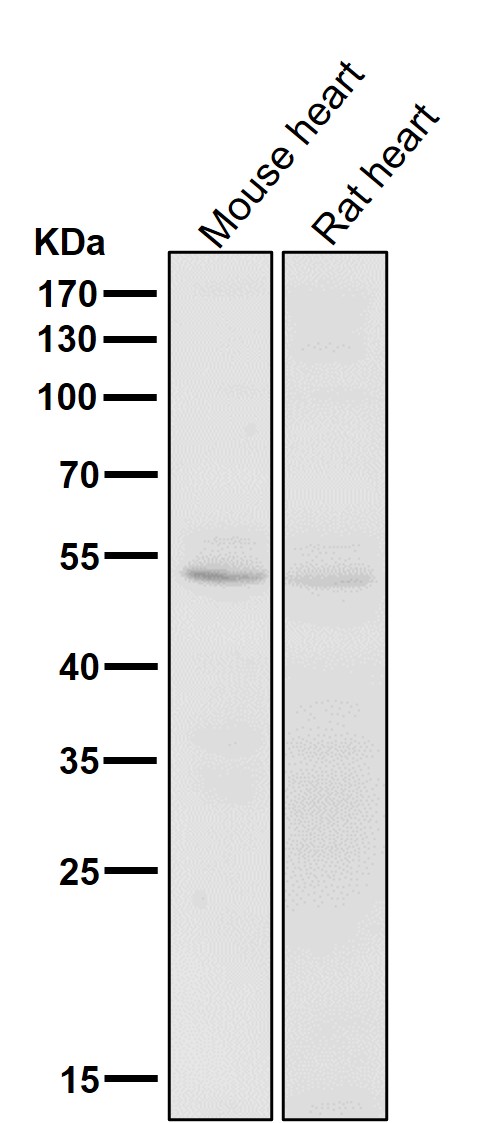
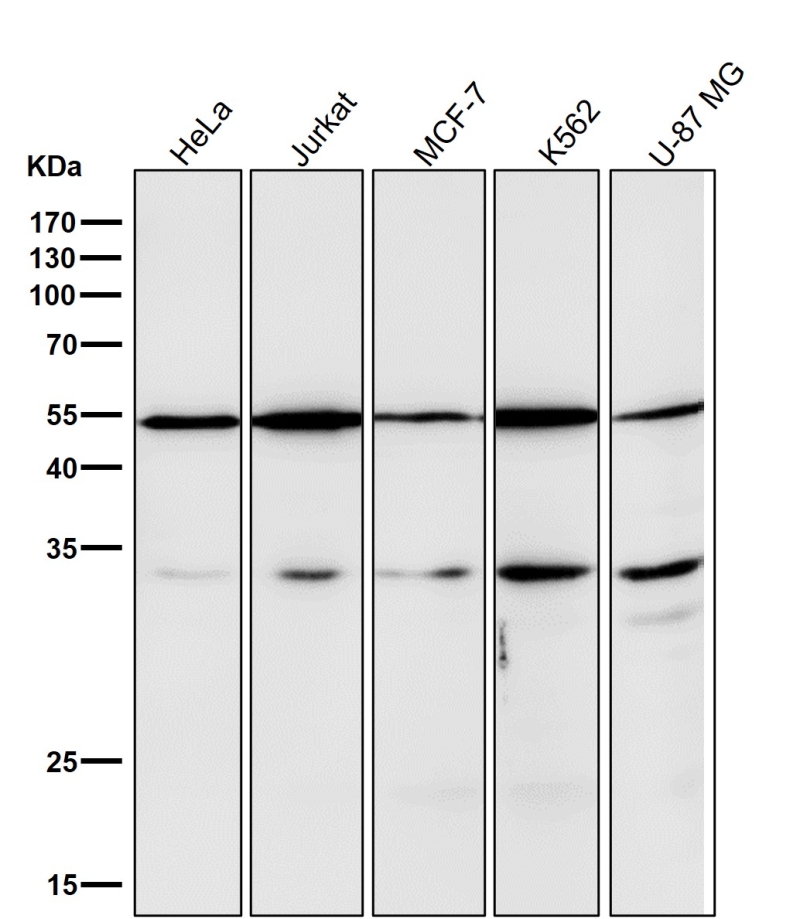
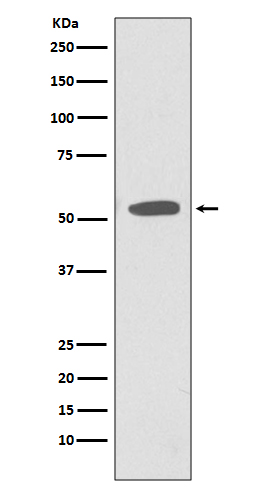
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 1/50-1/200 | Human,Mouse,Rat |
| FCM | 1/20-1/100 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | CD47; IAP; Integrin Associated Protein; MER6 ; OA3; Protein MER6 ; Antigen identified by monoclonal antibody 1D8;CD47 |
| WB Predicted band size | Calculated MW: 35 kDa ; Observed MW: 55 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | A synthesized peptide derived from human CD47 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.05% BSA and 50% glycerol. |
+ +
以下是关于CD47抗体的3篇代表性文献及其摘要概括:
---
1. **文献名称**:CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells
**作者**:Majeti R. et al. (2009)
**摘要**:该研究首次揭示CD47在急性髓系白血病(AML)干细胞中高表达,并与不良预后相关。抗CD47抗体可通过阻断CD47与巨噬细胞表面SIRPα的相互作用,增强巨噬细胞对白血病干细胞的吞噬作用,为靶向CD47的AML治疗提供理论基础。
---
2. **文献名称**:The CD47-signal regulatory protein alpha (SIRPα) interaction is a therapeutic target for human solid tumors
**作者**:Weiskopf K. et al. (2016)
**摘要**:研究证明CD47在多种实体瘤(如头颈癌、卵巢癌)中高表达,抗CD47抗体通过解除“别吃我”信号,激活巨噬细胞杀伤肿瘤细胞,并与放疗或化疗联合应用显著抑制肿瘤生长,提示其在实体瘤治疗中的潜力。
---
3. **文献名称**:Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma
**作者**:Chao M.P. et al. (2010)
**摘要**:本文发现抗CD47抗体与抗CD20单抗(利妥昔单抗)联用,可协同增强巨噬细胞对B细胞非霍奇金淋巴瘤细胞的吞噬作用,并在小鼠模型中实现肿瘤清除,为联合免疫治疗提供新策略。
---
4. **文献名称**:Structural and functional basis of the SIRPα-CD47 innate immune checkpoint
**作者**:Hatherley D. et al. (2015)
**摘要**:该研究解析了SIRPα-CD47相互作用的分子结构,揭示其调控巨噬细胞活性的机制,并设计出阻断该通路的新型抗体,为优化CD47靶向药物的特异性与安全性提供理论支持。
---
这些文献涵盖了CD47抗体在血液肿瘤、实体瘤中的治疗机制及临床前研究,以及分子结构层面的突破,反映了该领域的核心进展。
CD47. a transmembrane glycoprotein widely expressed on normal cells, serves as a critical "don't eat me" signal by binding to SIRPα on macrophages to inhibit phagocytosis. Cancer cells exploit this pathway by overexpressing CD47 to evade immune surveillance, making it a promising therapeutic target. CD47-blocking antibodies aim to disrupt this interaction, thereby enhancing macrophage-mediated phagocytosis of tumor cells and stimulating adaptive immune responses.
The development of CD47 antibodies faced challenges due to ubiquitous CD47 expression, particularly on red blood cells (RBCs) and platelets, leading to dose-limiting anemia and thrombocytopenia in early trials. To mitigate toxicity, next-generation agents employ strategies like preferential tumor targeting (e.g., tumor antigen bispecific antibodies) or engineered Fc regions to reduce RBC binding.
Clinical studies highlight potential in hematologic malignancies (e.g., myelodysplastic syndrome, lymphomas) and solid tumors, often in combination with chemotherapy, radiation, or checkpoint inhibitors (e.g., anti-PD-1/PD-L1). Preclinical data suggest synergistic effects, as CD47 blockade may reverse immunosuppressive microenvironments. However, patient stratification biomarkers and optimal dosing remain under investigation.
Despite hurdles, CD47-targeted therapies represent a novel immunotherapeutic approach, with ongoing trials exploring safety, efficacy, and combinatorial regimens to maximize antitumor activity while minimizing on-target toxicity.
×