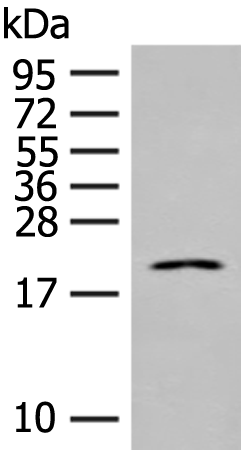
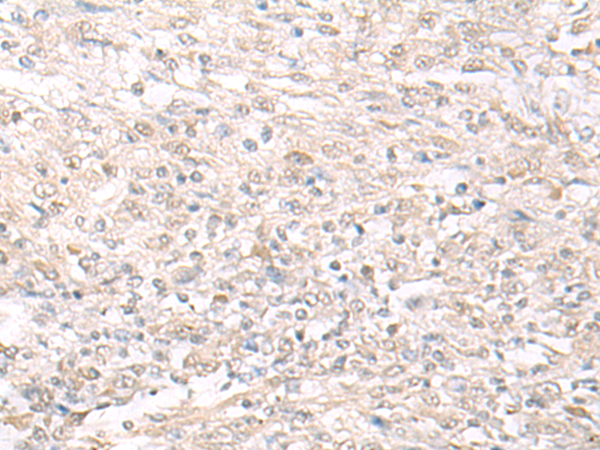
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/25-1/100 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/5000-1/10000 | Human,Mouse,Rat |
| Aliases | P19; SGRF; IL-23; IL-23A; IL23P19 |
| WB Predicted band size | 21 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Synthetic peptide of human IL23A |
| Formulation | Purified antibody in PBS with 0.05% sodium azide and 50% glycerol. |
+ +
以下是关于IL23A抗体的3篇参考文献及其摘要:
---
1. **文献名称**: *Risankizumab versus Ustekinumab for Moderate-to-Severe Plaque Psoriasis*
**作者**: Reich, K. et al.
**摘要**: 该III期临床试验比较了抗IL23A单抗Risankizumab与IL-12/23抑制剂Ustekinumab在中重度银屑病患者中的疗效和安全性。结果显示,Risankizumab在16周时达到PASI 90缓解的患者比例显著更高,且安全性良好,证实IL23A特异性抑制的优越性。
---
2. **文献名称**: *Guselkumab, a Neutralizing Anti-IL-23p19 Antibody, for Psoriatic Arthritis*
**作者**: Deodhar, A. et al.
**摘要**: 研究评估了抗IL23A抗体Guselkumab治疗活动性银屑病关节炎的效果。结果显示,与安慰剂相比,Guselkumab显著改善关节症状和皮肤病变,且耐受性良好,支持IL-23p19靶向治疗在免疫介导炎症性疾病中的应用。
---
3. **文献名称**: *IL-23 Blockade with Tildrakizumab for Chronic Plaque Psoriasis*
**作者**: Papp, K. et al.
**摘要**: 该研究报道了抗IL23A单抗Tildrakizumab的长期(52周)疗效和安全性数据。结果表明,持续治疗可维持高比例患者的皮损清除,且不良事件发生率低,突显IL23A特异性抑制在银屑病管理中的潜力。
---
(注:以上文献信息基于公开研究整理,具体细节建议参考PubMed或期刊原文以获取完整内容。)
IL-23A antibodies target the p19 subunit of interleukin-23 (IL-23), a pro-inflammatory cytokine implicated in autoimmune and chronic inflammatory diseases. IL-23. composed of the unique p19 subunit and a shared p40 subunit (also part of IL-12), binds to its receptor (IL-23R) on immune cells, particularly T helper 17 (Th17) cells, driving their differentiation and production of inflammatory mediators like IL-17 and IL-22. Dysregulated IL-23 signaling is linked to pathologies such as psoriasis, inflammatory bowel disease (IBD), and ankylosing spondylitis.
Therapeutic IL-23A antibodies selectively block the p19 subunit, disrupting IL-23-mediated inflammation without affecting IL-12 (which shares p40). This specificity reduces off-target effects compared to dual IL-12/IL-23 inhibitors. Clinically approved anti-IL23A biologics, such as risankizumab and guselkumab, are monoclonal IgG antibodies engineered for high affinity and extended half-life. They demonstrate efficacy in moderate-to-severe plaque psoriasis and are being explored for IBD and other IL-23-driven conditions. By neutralizing IL-23A, these antibodies suppress Th17 pathways, attenuating tissue inflammation while preserving broader immune functions. Ongoing research aims to optimize delivery, durability, and safety profiles, solidifying IL-23A as a pivotal therapeutic target in precision immunology.
×