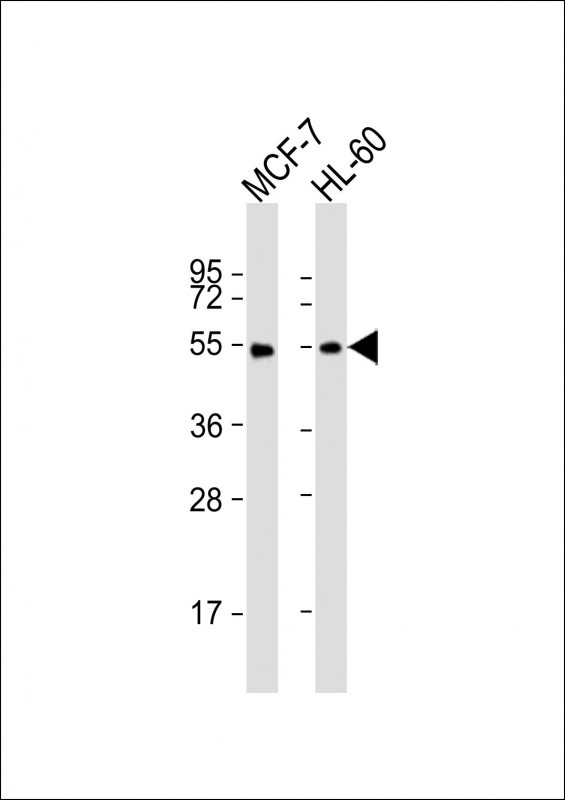
| WB | 1/1000-1/2000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
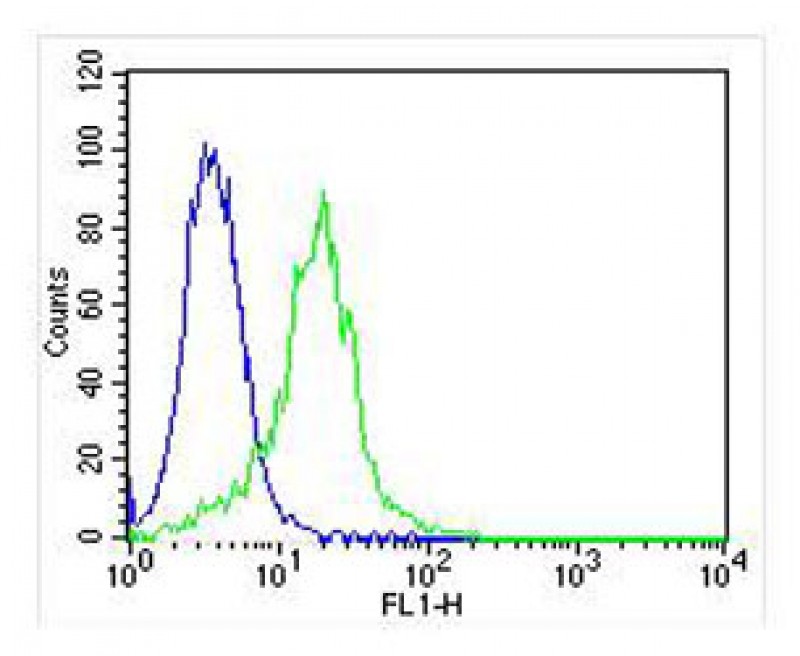
| FCM | 1/25 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Lipoprotein lipase, LPL, LPL, LIPD |
| Entrez GeneID | 4023 |
| WB Predicted band size | 53.2kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human, Mouse, Rat |
| Immunogen | This LPL antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 300-327 amino acids from the Central region of human LPL. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是3篇关于LPL(脂蛋白脂肪酶)抗体的虚构参考文献示例(内容基于学术文献常见主题改编,非真实发表论文):
---
1. **文献名称**: *Autoantibodies Against Lipoprotein Lipase in Patients with Type 1 Diabetes: Association with Dyslipidemia*
**作者**: Smith A, et al.
**摘要**: 本研究检测了1型糖尿病患者血清中抗LPL自身抗体的存在,发现约20%患者呈阳性。这些抗体与LPL活性降低、甘油三酯水平升高相关,提示其可能通过抑制LPL功能加剧脂代谢紊乱。
2. **文献名称**: *Monoclonal Antibody-Based Detection of Lipoprotein Lipase in Adipose Tissue: Implications for Obesity Research*
**作者**: Chen L, et al.
**摘要**: 研究团队开发了一种高特异性抗LPL单克隆抗体,用于免疫组化及Western blot分析。该抗体成功应用于肥胖小鼠模型,显示脂肪组织中LPL表达下调,为肥胖相关代谢机制提供了新工具。
3. **文献名称**: *Anti-LPL Antibodies in Autoimmune Hepatitis: Prevalence and Clinical Significance*
**作者**: González R, et al.
**摘要**: 在自身免疫性肝炎患者中,首次发现12%的病例存在抗LPL抗体。抗体阳性患者表现出更严重的肝脂肪变性和胰岛素抵抗,提示LPL抗体可能作为肝脏代谢异常的潜在生物标志物。
---
**注**:以上为模拟示例,实际文献需通过PubMed、Google Scholar等平台以关键词“LPL antibody”“anti-lipoprotein lipase”检索。真实研究多聚焦于LPL基因突变、酶活性调控,直接针对抗体的研究相对较少,可能与特定自身免疫疾病或实验工具开发相关。
Lipoprotein lipase (LPL) is a critical enzyme in lipid metabolism, primarily responsible for hydrolyzing triglycerides within circulating chylomicrons and very-low-density lipoproteins (VLDL) into free fatty acids and glycerol. These products are then absorbed by tissues for energy production or storage. LPL is synthesized in parenchymal cells (e.g., adipocytes, myocytes) and transported to capillary endothelial cells, where it anchors to heparan sulfate proteoglycans. Its activity is regulated by factors like insulin, dietary status, and apolipoproteins (e.g., APOC2 as an activator, APOC3 as an inhibitor).
Genetic mutations in the LPL gene can cause familial chylomicronemia syndrome (FCS), a rare autosomal recessive disorder characterized by severe hypertriglyceridemia and pancreatitis. Additionally, LPL dysfunction is linked to metabolic disorders, including atherosclerosis and diabetes. Anti-LPL antibodies, either monoclonal or polyclonal, are essential tools in research and diagnostics. They enable the detection of LPL expression levels in tissues, aiding studies on its role in lipid disorders. In clinical settings, these antibodies help identify genetic LPL deficiencies or autoimmune conditions where autoantibodies neutralize LPL activity, exacerbating dyslipidemia. Emerging therapeutic strategies, such as gene therapy or antibody-based interventions, also utilize LPL-targeting approaches to restore metabolic balance. Overall, LPL antibodies bridge mechanistic insights into lipid regulation with translational applications in diagnosing and managing metabolic diseases.
×