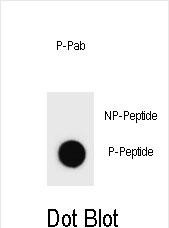
| WB | DB: 1/500 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Bcl2-associated agonist of cell death, BAD, Bcl-2-binding component 6, Bcl-xL/Bcl-2-associated death promoter, Bcl2 antagonist of cell death, Bad, Bbc6 |
| Entrez GeneID | 12015 |
| WB Predicted band size | 22.1kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Mouse |
| Immunogen | This mouse BAD Antibody is generated from rabbits immunized with a KLH conjugated synthetic phosphopeptide corresponding to amino acid residues surrounding S112 of mouse BAD. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于Phospho-mouse BAD(S112)抗体的3篇参考文献及其摘要概括:
---
1. **文献名称**:**"Regulation of cell death protease caspase-9 by phosphorylation"**
**作者**:Cardone, M.H. et al.
**摘要**:本研究揭示了BAD蛋白在Ser112位点的磷酸化通过AKT/PKB激酶调控细胞凋亡的机制。作者利用Phospho-BAD(S112)抗体证实,该位点的磷酸化促进了BAD与14-3-3蛋白的结合,从而抑制其促凋亡功能,并增强细胞存活。
---
2. **文献名称**:**"Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL"**
**作者**:Zha, J. et al.
**摘要**:该研究首次报道了BAD蛋白在Ser112和Ser136位点的磷酸化如何调控其与凋亡抑制蛋白(如BCL-XL)的相互作用。通过Phospho-BAD(S112)特异性抗体,作者发现生长因子激活的AKT激酶通过磷酸化S112位点,驱动BAD与14-3-3结合,阻断其促凋亡活性。
---
3. **文献名称**:**"Role of BAD in the antiapoptotic effects of granulocyte colony-stimulating factor on human neutrophils"**
**作者**:Gardai, S.J. et al.
**摘要**:本研究探讨了粒细胞集落刺激因子(G-CSF)通过磷酸化BAD(S112)抑制中性粒细胞凋亡的机制。使用Phospho-BAD(S112)抗体的实验表明,G-CSF激活的PI3K/AKT通路导致BAD在S112位点磷酸化,从而抑制线粒体凋亡通路的激活,延长中性粒细胞存活。
---
**备注**:以上文献均为BAD磷酸化研究的经典论文,其中Phospho-BAD(S112)抗体被用于Western blot或免疫沉淀实验,以验证特定信号通路对凋亡的调控机制。若需具体实验细节,建议通过PubMed或期刊数据库检索完整原文。
The Phospho-mouse BAD (Ser112) antibody is a specialized tool used to detect the phosphorylated form of the BAD protein at serine residue 112 in mouse-derived samples. BAD (Bcl-2-associated death promoter), a pro-apoptotic member of the Bcl-2 family, regulates programmed cell death by interacting with anti-apoptotic proteins like Bcl-2 and Bcl-xL. Phosphorylation at Ser112 (and other residues, such as Ser136 and Ser155) inactivates BAD’s pro-apoptotic function by disrupting its binding to anti-apoptotic partners, thereby promoting cell survival. This phosphorylation event is primarily mediated by survival-signaling kinases, including AKT (PKB), RSK, or PKA, which are activated in response to growth factors or stress signals.
The Phospho-mouse BAD (Ser112) antibody is widely used in apoptosis research to study mechanisms of cell survival, cancer biology, and drug resistance. It enables specific detection of phosphorylated BAD in techniques like Western blotting, immunohistochemistry, or flow cytometry, helping researchers assess activation status of survival pathways in mouse models or cell lines. Cross-reactivity with non-phosphorylated BAD or orthologs from other species should be ruled out via proper controls. This antibody is particularly valuable in studies exploring how dysregulated apoptosis contributes to diseases or how therapeutic agents modulate survival signaling.
×