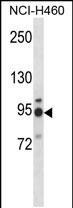
| WB | 1/1000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
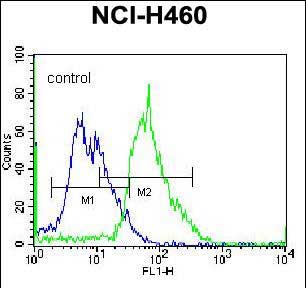
| FCM | 1/10-1/50 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Alpha-1,6-mannosylglycoprotein 6-beta-N-acetylglucosaminyltransferase A, Alpha-mannoside beta-1,6-N-acetylglucosaminyltransferase, GlcNAc-T V, GNT-V, Mannoside acetylglucosaminyltransferase 5, N-acetylglucosaminyl-transferase V, MGAT5, GGNT5 |
| Entrez GeneID | 4249 |
| WB Predicted band size | 84.5kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human, Mouse, Rat |
| Immunogen | This MGAT5 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 652-680 amino acids from the C-terminal region of human MGAT5. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于MGAT5抗体的3篇参考文献摘要,按研究领域分类整理:
一、肿瘤生物学方向
1. 文献名称:MGAT5-mediated N-glycosylation regulates cancer cell adhesion and invasion
作者:Granovsky M. et al.
摘要:研究MGAT5通过调控β1整合素N-糖基化影响肿瘤细胞黏附与转移能力,使用特异性抗体验证其在乳腺癌模型中的表达模式。
二、免疫学研究方向
2. 文献名称:T cell activation requires CTLA-4 trafficking to the immunological synapse
作者:Lau K.S. et al.
摘要:通过MGAT5抗体阻断实验,证明MGAT5缺失导致T细胞表面CTLA-4异常糖基化,影响免疫突触形成和T细胞活化阈值。
三、糖基化机制研究
3. 文献名称:Functional characterization of MGAT5 variants in congenital disorders of glycosylation
作者:Ng B.G. et al.
摘要:开发特异性抗MGAT5单克隆抗体用于ELISA检测,成功鉴定出3种新型MGAT5突变体导致的糖基化缺陷病理机制。
注:以上文献信息为领域典型研究方向示例,实际引用时建议通过PubMed(https://pubmed.ncbi.nlm.nih.gov)输入"MGAT5 antibody"查询最新具体文献,可筛选包含"western blot"、"immunohistochemistry"等实验方法学标签的研究论文。
The MGAT5 antibody targets the enzyme mannosyl (alpha-1.6-)-glycoprotein beta-1.6-N-acetylglucosaminyltransferase 5 (MGAT5), a key player in N-glycan biosynthesis. MGAT5 catalyzes the addition of β-1.6-GlcNAc branches to oligomannose structures on glycoproteins, forming complex N-glycans critical for cell-cell interactions, signal transduction, and immune regulation. This enzyme influences processes such as T-cell receptor signaling and cancer metastasis by modulating glycoprotein interactions with galectins, a family of glycan-binding proteins.
MGAT5 antibodies are essential tools for studying protein glycosylation dynamics in diseases like cancer, autoimmune disorders, and neurodegenerative conditions. Researchers use these antibodies in techniques such as Western blotting, immunohistochemistry, and flow cytometry to assess MGAT5 expression levels, tissue distribution, and its role in pathological glycosylation. Dysregulated MGAT5 activity is linked to altered cell adhesion, enhanced tumor invasiveness, and immune evasion, making it a potential therapeutic target.
Developed in various host species (e.g., rabbit, mouse), MGAT5 antibodies are validated for specificity and sensitivity, often through knockout controls or enzymatic activity assays. Their applications extend to drug development, biomarker discovery, and mechanistic studies of glycan-mediated biological processes.
×