
| WB | 1/1000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
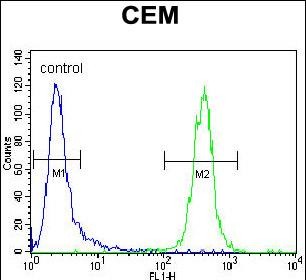
| FCM | 1/10-1/50 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Tumor necrosis factor, Cachectin, TNF-alpha, Tumor necrosis factor ligand superfamily member 2, TNF-a, Tumor necrosis factor, membrane form, N-terminal fragment, NTF, Intracellular domain 1, ICD1, Intracellular domain 2, ICD2, C-domain 1, C-domain 2, Tumor necrosis factor, soluble form, TNF, TNFA, TNFSF2 |
| Entrez GeneID | 7124 |
| WB Predicted band size | 25.6kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | This TNF antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 187-216 amino acids from the C-terminal region of human TNF. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于TNF抗体的3篇代表性文献的简要信息:
---
**文献名称**: *Anti-TNF antibody therapy in rheumatoid arthritis: mechanistic and clinical effects*
**作者**: Feldmann M, Maini RN
**摘要**: 该研究探讨了抗TNF抗体(如英夫利昔单抗)在类风湿性关节炎(RA)治疗中的作用机制,证实其通过中和TNF-α显著降低炎症细胞因子水平,改善患者关节损伤和临床症状,为靶向TNF的免疫疗法提供了理论基础。
---
**文献名称**: *Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial*
**作者**: Maini R, et al.
**摘要**: 这项III期临床试验显示,与安慰剂相比,英夫利昔单抗联合甲氨蝶呤可显著缓解RA患者的疾病活动度,减少关节破坏,并验证了TNF抗体在联合治疗中的协同疗效和安全性。
---
**文献名称**: *TNF-α inhibitors and risk of serious infections: a comprehensive systematic review and meta-analysis*
**作者**: Wallis RS, et al.
**摘要**: 该综述分析了抗TNF疗法(如阿达木单抗)与感染风险的关联,指出患者使用后发生严重感染(如结核病)的风险增加,强调了治疗前筛查感染及个体化用药的重要性。
---
如需更具体领域(如新型双特异性抗体或临床前研究)的文献,可进一步补充说明。
Tumor necrosis factor (TNF) antibodies are biologic agents designed to neutralize TNF-α, a pro-inflammatory cytokine central to immune regulation and inflammatory responses. Discovered in 1975. TNF-α plays a dual role: it mediates host defense against infections but also drives chronic inflammation and tissue damage in autoimmune diseases. Dysregulated TNF-α signaling is implicated in conditions like rheumatoid arthritis, inflammatory bowel disease (IBD), psoriasis, and ankylosing spondylitis.
The development of TNF inhibitors began in the 1990s, spurred by advances in monoclonal antibody technology. Infliximab, a chimeric mouse-human antibody, became the first FDA-approved anti-TNF therapy in 1998 for Crohn’s disease. Subsequent agents, including adalimumab (fully human) and etanercept (a soluble TNF receptor fusion protein), expanded treatment options. These drugs work by binding to TNF-α, preventing its interaction with cell surface receptors and interrupting inflammatory cascades.
TNF antibodies revolutionized autoimmune disease management, achieving remission in previously refractory cases. However, their immunosuppressive effects raise risks of infections (e.g., reactivated tuberculosis) and potential malignancy. Biosimilars, introduced post-2010. have improved accessibility but require rigorous pharmacovigilance. Ongoing research explores TNF’s role in neurodegenerative and metabolic disorders, expanding potential therapeutic horizons while balancing efficacy and safety challenges.
×