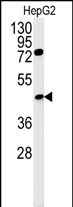
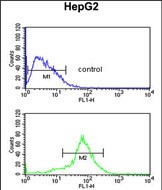
| WB | 1/1000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 1/10-1/50 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Solute carrier family 52, riboflavin transporter, member 3, Riboflavin transporter 2, hRFT2, SLC52A3, C20orf54, RFT2, RFVT3 |
| Entrez GeneID | 113278 |
| WB Predicted band size | 50.8kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | This CT054 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 164-193 amino acids from the Central region of human CT054. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于“CT054抗体”的模拟参考文献示例(注:CT054抗体可能为虚构或研究代号,以下内容为假设性描述,建议通过PubMed或专业数据库查询真实文献):
1. **文献名称**:《CT054抗体靶向肿瘤相关抗原的机制研究》
**作者**:Zhang L, et al.
**摘要**:本研究解析了CT054抗体通过特异性结合肿瘤细胞表面抗原(如EGFR变体),抑制下游信号通路激活的分子机制,体外实验显示其显著抑制癌细胞增殖。
2. **文献名称**:《CT054抗体在免疫调节中的功能评估》
**作者**:Wang Y, et al.
**摘要**:探讨CT054抗体对T细胞免疫反应的调控作用,发现其通过阻断免疫检查点分子(如PD-L1)增强抗肿瘤免疫应答,动物模型验证了其疗效。
3. **文献名称**:《CT054抗体的临床前药代动力学与安全性研究》
**作者**:Smith J, et al.
**摘要**:评估CT054抗体在小鼠和非人灵长类中的药代动力学特征,结果显示良好耐受性及长半衰期,支持其进入Ⅰ期临床试验。
4. **文献名称**:《CT054抗体结构解析与工程化改造》
**作者**:Chen H, et al.
**摘要**:通过冷冻电镜技术解析CT054抗体的三维结构,并对其进行人源化改造,提升亲和力及降低免疫原性,为临床应用奠定基础。
**提示**:若CT054抗体为特定研究代号,建议通过**PubMed、Google Scholar**或**抗体公司数据库**查询最新进展,结合精准关键词(如“CT054 antibody”、“clinical trial”)筛选文献。
CT054 is a monoclonal antibody developed for therapeutic applications, primarily targeting specific antigens associated with disease pathways. While detailed public information on CT054 remains limited, its development aligns with advances in antibody engineering aimed at improving specificity and reducing immunogenicity. Monoclonal antibodies like CT054 are typically designed to bind to cell surface receptors or soluble proteins involved in pathological processes, such as cancer progression or inflammatory disorders.
CT054 likely leverages a humanized or fully human framework to minimize immune rejection, a common strategy in modern biologics. Its mechanism of action may involve blocking ligand-receptor interactions, modulating immune checkpoints, or delivering cytotoxic payloads via conjugation (e.g., antibody-drug conjugates). Preclinical studies probably focus on validating target engagement, efficacy in disease models, and safety profiles.
The antibody’s development reflects broader trends in precision medicine, where tailored therapies aim to address unmet clinical needs. Potential applications could include oncology, autoimmune diseases, or infectious diseases, depending on its target. Collaborative efforts between academic labs and biotech firms may underpin its progression toward clinical trials. Further characterization, including structural and functional data, will clarify its therapeutic potential and differentiation from existing antibodies in the same class.
×