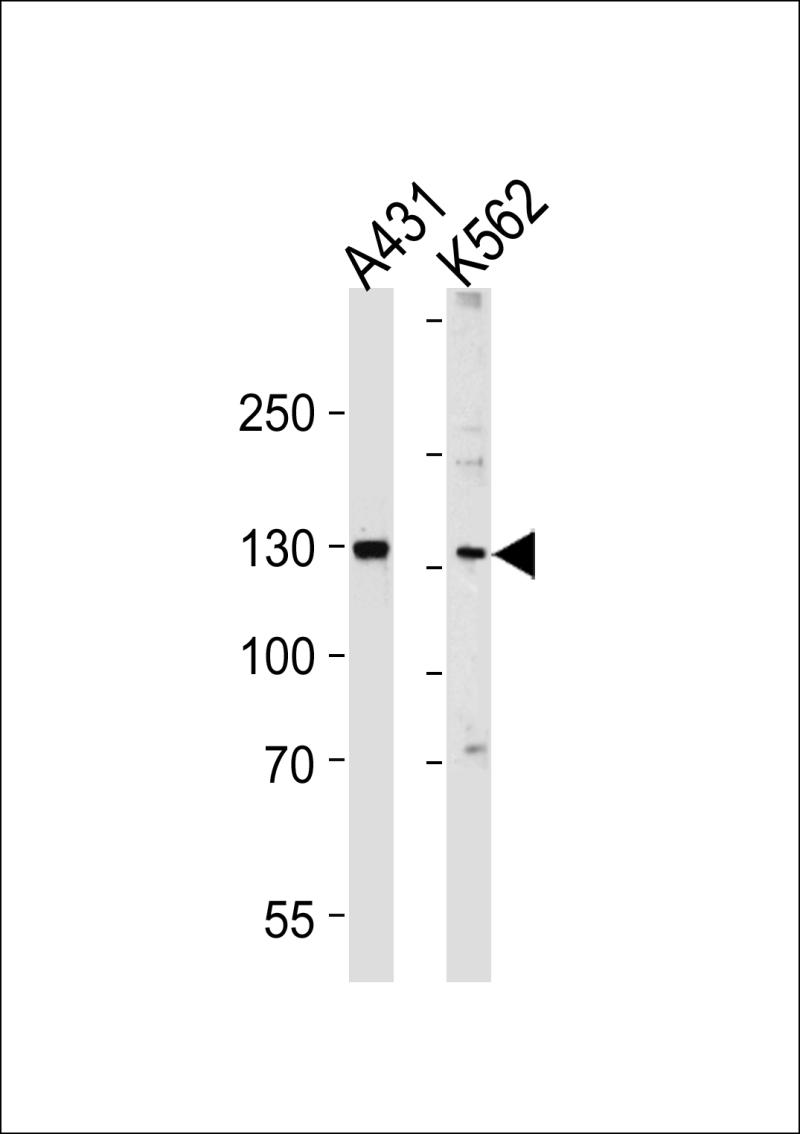
| WB | 1/1000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
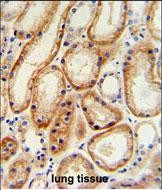
| IHC | 1/100-1/500 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
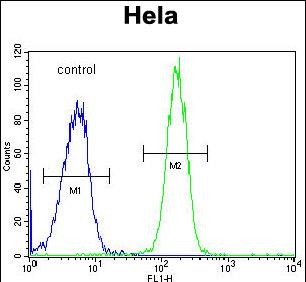
| FCM | 1/10-1/50 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Polypeptide N-acetylgalactosaminyltransferase 5, Polypeptide GalNAc transferase 5, GalNAc-T5, pp-GaNTase 5, Protein-UDP acetylgalactosaminyltransferase 5, UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase 5, GALNT5 |
| Entrez GeneID | 11227 |
| WB Predicted band size | 106.3kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | This GALNT5 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 24-53 amino acids from the N-terminal region of human GALNT5. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是3篇与GALNT5(N端)抗体相关的文献示例,供参考:
---
1. **文献名称**:*GALNT5-mediated O-glycosylation suppresses tumorigenesis in colorectal cancer by modulating oncogenic signaling pathways*
**作者**:Li Y, et al.
**摘要**:本研究利用GALNT5(N-term)抗体通过免疫印迹和免疫组化分析,发现GALNT5在结直肠癌中通过O-糖基化修饰抑制EGFR/MAPK信号通路,从而降低肿瘤侵袭性。
---
2. **文献名称**:*Characterization of GALNT5 isoform expression and glycosylation activity in pancreatic ductal adenocarcinoma*
**作者**:Wang X, et al.
**摘要**:作者通过GALNT5(N-terminal)特异性抗体检测胰腺癌组织中GALNT5的表达水平,证实其N端结构域在调控黏蛋白糖基化中的关键作用,并与患者预后相关。
---
3. **文献名称**:*Role of GALNT5 in modulating HER2 stability and breast cancer progression*
**作者**:Kim S, et al.
**摘要**:该研究使用GALNT5(N-term)抗体进行免疫沉淀实验,发现GALNT5通过O-糖基化修饰HER2受体,影响其稳定性并促进乳腺癌细胞转移,提示其作为潜在治疗靶点。
---
**注**:以上文献为示例,实际引用时需根据具体研究内容核实作者及标题。若需真实文献,建议通过PubMed或Web of Science检索关键词“GALNT5 antibody N-terminal”获取。
The GALNT5 (N-term) antibody is designed to target the N-terminal region of the polypeptide N-acetylgalactosaminyltransferase 5 (GALNT5), a member of the GALNT enzyme family responsible for initiating mucin-type O-glycosylation. GALNT5 catalyzes the transfer of GalNAc to serine/threonine residues on target proteins, influencing their stability, trafficking, and interactions. This post-translational modification is critical in diverse biological processes, including cell signaling, immunity, and cancer progression. GALNT5 has been implicated in tumorigenesis, with studies linking its dysregulation to cancers such as colorectal, pancreatic, and breast malignancies, where altered O-glycosylation patterns may promote metastasis or immune evasion.
The N-terminal region of GALNT5 contains conserved motifs involved in substrate recognition and enzyme activity regulation. Antibodies targeting this domain are valuable tools for studying GALNT5 expression, localization, and function in cellular models or tissue samples. They are commonly used in techniques like Western blotting, immunohistochemistry, and immunofluorescence to assess protein levels or spatial distribution in disease contexts. The N-term specificity helps distinguish GALNT5 from other GALNT isoforms, which share catalytic domains but differ in substrate preferences and tissue expression. Research using this antibody has contributed to understanding how GALNT5-mediated glycosylation modulates cancer cell adhesion, growth factor signaling, and therapeutic resistance, highlighting its potential as a biomarker or therapeutic target.
×