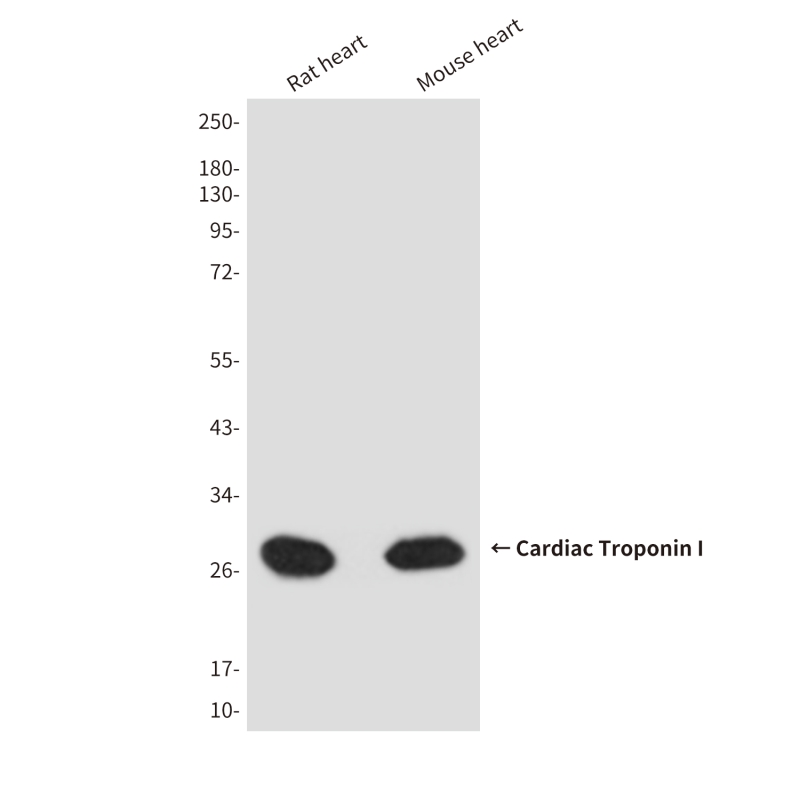
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
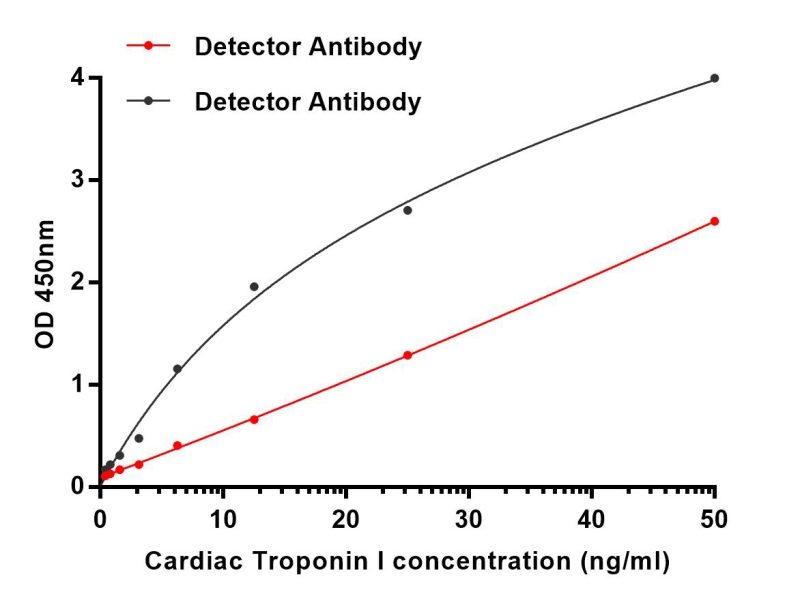
| Elisa | 1/10000 | Human,Mouse,Rat |
| Aliases | TNNI3; TNNC1; Troponin I; cardiac muscle; Cardiac troponin I |
| Entrez GeneID | 7137 |
| clone | 7G3 |
| WB Predicted band size | Calculated MW: 24 kDa; Observed MW: 28 kDa |
| Host/Isotype | Mouse IgG2a |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse,Rat |
| Immunogen | Synthetic peptides corresponding to the sequence of human Cardiac Troponin I. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.5%BSA and 50% glycerol. |
+ +
以下是3篇关于心肌肌钙蛋白I(cTnI)抗体的代表性文献及其摘要概括:
---
1. **"Characterization of monoclonal antibodies for cardiac troponin I and preliminary results in immunoassay"**
*By Bodor GS, Porter S, Landt Y, Ladenson JH*
该研究报道了针对cTnI不同表位的单克隆抗体的开发与表征,验证了其在夹心法免疫检测中的高特异性,为心肌梗死的血清学诊断奠定了基础。
2. **"Development and validation of a sensitive sandwich ELISA for cardiac troponin I"**
*By Katus HA, Remppis A, Scheffold T*
研究通过优化两种高亲和力抗cTnI单克隆抗体的组合,建立了一种高灵敏度的ELISA检测方法,显著提高了对心肌损伤早期诊断的敏感性。
3. **"Antibody selection for point-of-care cardiac troponin I assays"**
*By Apple FS, Wu AH, Jaffe AS*
文章分析了不同抗体表位选择对即时检测(POCT)设备性能的影响,强调表位稳定性对临床结果一致性的重要性,推动检测标准化的建立。
---
以上文献聚焦于cTnI抗体的开发、检测方法优化及临床应用挑战,反映了该领域的关键研究方向。如需具体年份或期刊信息可进一步补充。
Cardiac Troponin I (cTnI) is a highly specific biomarker for myocardial injury, exclusively expressed in cardiac muscle. Its detection in blood is critical for diagnosing acute coronary syndromes, particularly myocardial infarction. Antibodies targeting cTnI form the basis of modern diagnostic assays, enabling precise measurement even at low concentrations (ng/L range).
cTnI exists as a ternary complex with Troponin C and T in healthy myocardium. During injury, proteolytic cleavage releases free cTnI into circulation. Its unique 31-amino acid N-terminal extension distinguishes it from skeletal muscle isoforms, making this region a common target for antibody development. Most commercial assays employ paired monoclonal antibodies in sandwich ELISA formats – one captures cTnI while the other detects epitopes in the stable central domain (residues 30-110), avoiding degradation-prone terminal regions.
Key challenges in antibody design include ensuring specificity against skeletal Troponin I, accounting for post-translational modifications (phosphorylation, proteolysis), and overcoming interference from heterophilic antibodies. Recent advances focus on developing high-sensitivity assays (hs-cTnI) that detect >99th percentile of healthy populations, enabling earlier diagnosis. Standardization remains an issue due to varying antibody epitope targets across manufacturers.
Emerging research explores cTnI antibody applications beyond diagnostics, including prognostic stratification and therapy monitoring. Novel antibody engineering techniques aim to improve stability in point-of-care devices and enhance cross-platform reproducibility.
×