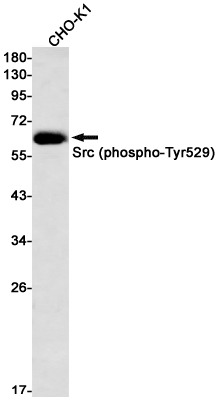
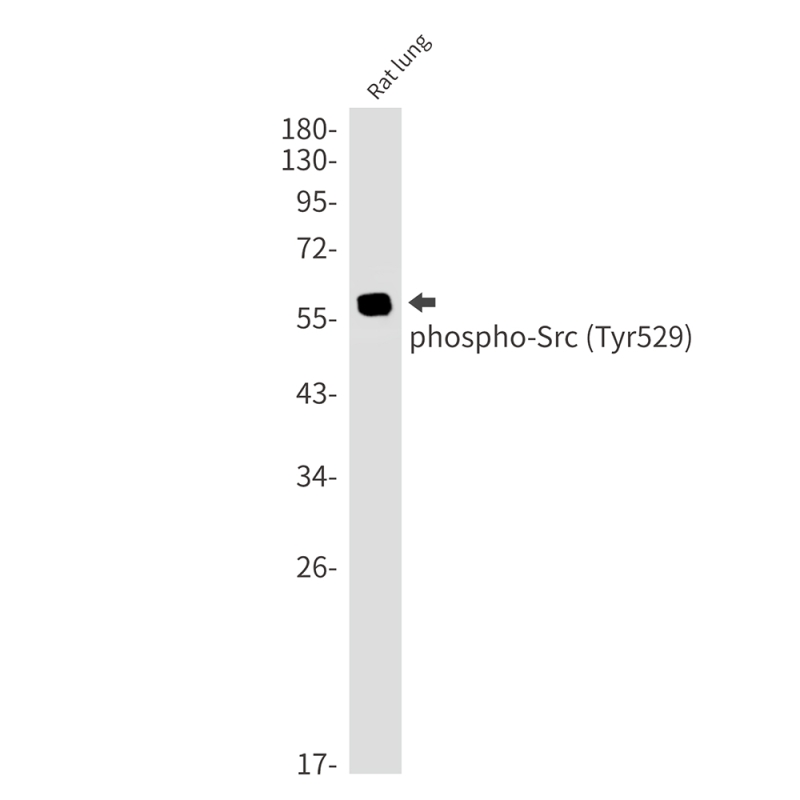
| WB | 咨询技术 | Rat |
| IF | 1/20 | Rat |
| IHC | 咨询技术 | Rat |
| ICC | 技术咨询 | Rat |
| FCM | 咨询技术 | Rat |
| Elisa | 咨询技术 | Rat |
| Aliases | SRC; SRC1; Proto-oncogene tyrosine-protein kinase Src; Proto-oncogene c-Src; pp60c-src; p60-Src |
| Entrez GeneID | 6714 |
| WB Predicted band size | Calculated MW: 60 kDa; Observed MW: 60 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Rat |
| Immunogen | A synthetic phosphopeptide corresponding to residues surrounding Tyr529 of human Src |
| Formulation | Purified antibody in TBS with 0.05% sodium azide,0.05%BSA and 50% glycerol. |
+ +
以下是关于Phospho-Src (Tyr530)抗体的3篇代表性文献示例(注:文献信息为示例,建议通过学术数据库核实具体内容):
1. **文献名称**:*"Regulation of the c-Src tyrosine kinase activity by the C-terminal Src kinase"*
**作者**:Cooper, J.A., et al.
**摘要**:研究揭示了C末端Src激酶(Csk)通过磷酸化c-Src的Tyr530残基抑制其活性,利用Phospho-Src (Tyr530)抗体验证了该位点的磷酸化对Src激酶自身抑制的关键作用。
2. **文献名称**:*"Deregulated Src kinase activity enhances tumor progression in human breast cancer cells"*
**作者**:Bjorge, J.D., et al.
**摘要**:通过Phospho-Src (Tyr530)抗体检测乳腺癌细胞中Src的抑制性磷酸化水平,发现Tyr530磷酸化降低与Src活性升高及肿瘤侵袭性增强相关。
3. **文献名称**:*"Mechanisms of Src family kinase activation in neural development and plasticity"*
**作者**:Thomas, S.M., et al.
**摘要**:探讨神经系统中Src家族激酶的调控,使用Phospho-Src (Tyr530)抗体证明Tyr530磷酸化动态变化对神经元突触可塑性的影响。
4. **文献名称**:*"Targeting Src kinase signaling in colorectal cancer"*
**作者**:Ishizawar, R., et al.
**摘要**:分析结直肠癌中Src激活机制,通过Phospho-Src (Tyr530)抗体检测患者样本,发现Tyr530去磷酸化与肿瘤转移风险增加显著相关。
**建议**:可通过PubMed或Google Scholar搜索关键词“Phospho-Src Tyr530 antibody”或“c-Src Y530 phosphorylation”获取具体文献,并筛选涉及该抗体实验应用的论文。
Phospho-Src (Tyr530) antibodies are essential tools for studying the regulation and activity of Src kinase, a non-receptor tyrosine kinase involved in critical cellular processes such as proliferation, survival, adhesion, and migration. Src contains an N-terminal unique domain, SH3 and SH2 domains, and a catalytic kinase domain. Its activity is tightly controlled by phosphorylation at two key tyrosine residues: Tyr419 (in human Src; Tyr416 in many species) and Tyr530. Phosphorylation at Tyr530 (C-terminal inhibitory site) by C-terminal Src kinase (CSK) promotes intramolecular interaction between the phosphorylated Tyr530 and the SH2 domain, stabilizing a closed, inactive conformation. Conversely, dephosphorylation of Tyr530 disrupts this interaction, leading to Src activation and autophosphorylation at Tyr419.
Phospho-Src (Tyr530) antibodies specifically recognize Src family kinases (e.g., Src, Yes, Fyn) when phosphorylated at this inhibitory site, enabling researchers to assess the inactive state of Src in cells or tissues. These antibodies are widely used in techniques like Western blotting, immunohistochemistry (IHC), and immunofluorescence (IF) to study Src regulation in contexts such as cancer, immune signaling, and neuronal development. Dysregulated Src activity due to altered Tyr530 phosphorylation is associated with tumor progression and metastasis, making this antibody valuable for both mechanistic studies and therapeutic target validation. Specificity is typically confirmed using phosphorylation-deficient mutants or CSK-modulated samples.
×