| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 1/20-1/50 | Human,Mouse,Rat |
| IHC | 1/100-1/200 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 1/20-1/100 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | EGLN4 ; HIFPH4; Hypoxia inducible factor prolyl 4 hydroxylase; P4H with transmembrane domain; P4htm; PH4; PHD4; Proline 4 hydroxylase; Prolyl hydroxlase domain containing 4;;HIF PH4 |
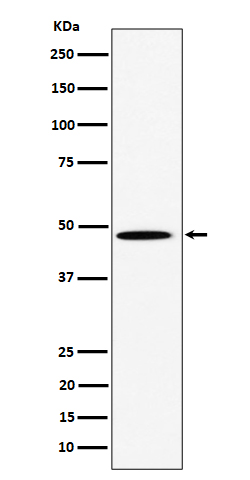
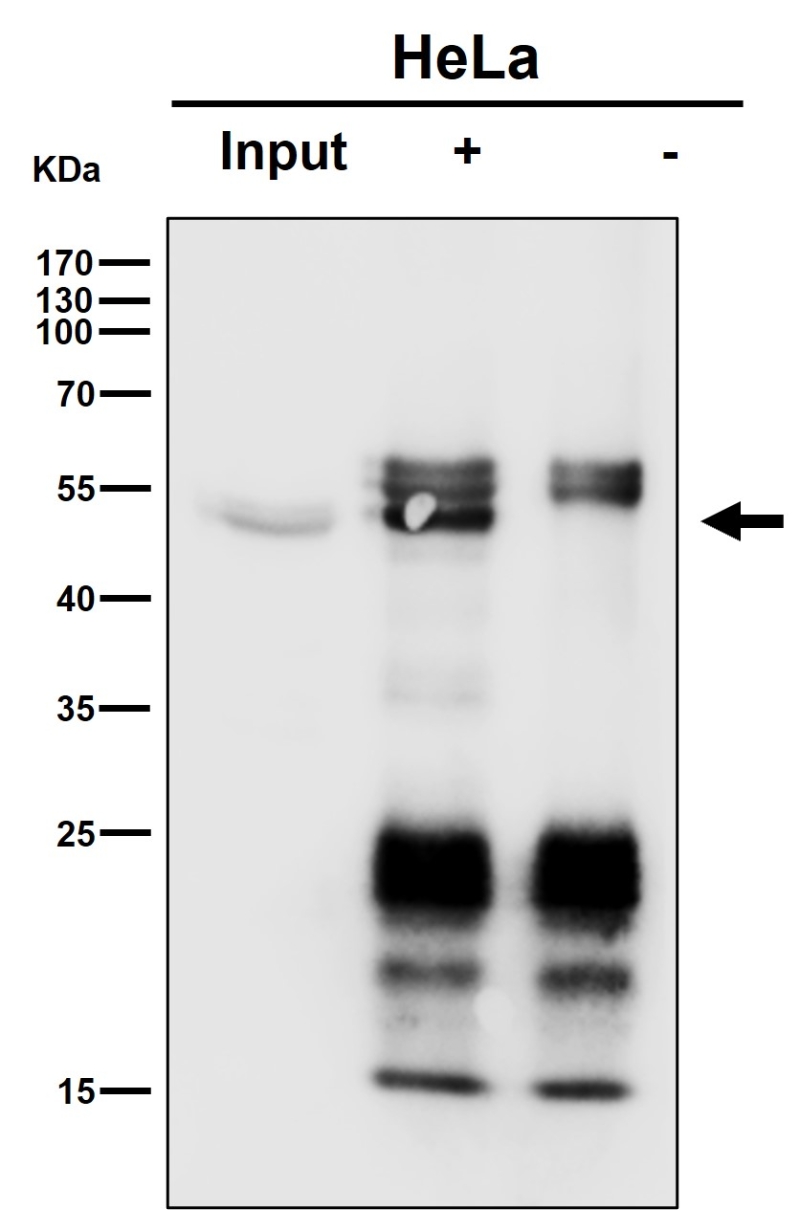
| WB Predicted band size | Calculated MW: 57 kDa ; Observed MW: 47 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | A synthesized peptide derived from human HIF PH4 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide,0.05% BSA and 50% glycerol. |
+ +
以下是3篇关于HIF Prolyl Hydroxylases(PHDs)抗体的参考文献,按研究方向和抗体应用场景分类:
---
### 1. **文献名称**:*"C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation"*
**作者**:Epstein, A. C. R., et al. (2001)
**摘要**:该研究首次阐明HIF Prolyl Hydroxylases(PHDs)通过羟化HIF-α亚基调控其稳定性。文中使用兔多克隆抗体检测PHD2(EGLN1)在细胞中的表达,验证了其在低氧条件下对HIF-1α的降解作用,为后续抗体在缺氧机制研究中的应用奠定基础。
---
### 2. **文献名称**:*"Regulation of hypoxia-inducible mRNA expression via oxygen-dependent protein stability"*
**作者**:Bruick, R. K., & McKnight, S. L. (2001)
**摘要**:研究通过免疫印迹(Western blot)和免疫沉淀(IP)技术,验证了针对PHD1(EGLN2)的小鼠单克隆抗体的特异性。作者发现PHD1在常氧条件下通过羟化HIF-1α的Pro564位点促进其泛素化降解,抗体数据支持了PHD酶的氧敏感功能。
---
### 3. **文献名称**:*"PHD3 regulates differentiation and lipid metabolism in adipocytes via hydroxylation-independent mechanisms"*
**作者**:Takeda, K., et al. (2006)
**摘要**:该文利用抗PHD3(EGLN3)的山羊多克隆抗体,在基因敲除小鼠模型中验证了抗体的特异性。研究发现,PHD3不仅通过羟化HIF调控代谢,还可能通过非羟化依赖途径影响脂肪细胞分化,抗体在组织切片染色中显示了PHD3的亚细胞定位。
---
### 应用场景扩展:
- **疾病研究**:上述抗体被广泛用于肿瘤、缺血性疾病中HIF通路的机制研究(如Hirota et al., *Cancer Cell* 2004)。
- **验证工具**:部分抗体被商业化(如Cell Signaling Technology的#PHD2抗体),用于检测PHDs的蛋白表达水平和酶活性。
如需更详细的方法学(如抗体稀释比例、交叉反应性数据),可进一步提供具体文献。
HIF prolyl hydroxylases (PHDs), also known as EglN enzymes, are oxygen-sensitive enzymes central to cellular oxygen sensing. They regulate the stability of hypoxia-inducible factors (HIFs), transcription factors that mediate adaptive responses to hypoxia. Under normoxic conditions, PHDs hydroxylate specific proline residues on HIF-α subunits (HIF-1α, HIF-2α, HIF-3α), marking them for proteasomal degradation via the von Hippel-Lindau (VHL) E3 ubiquitin ligase complex. During hypoxia, PHD activity decreases, allowing HIF-α to accumulate, dimerize with HIF-β, and activate genes involved in angiogenesis, erythropoiesis, and metabolic reprogramming.
Antibodies targeting PHDs (PHD1. PHD2. PHD3) are critical tools for studying their expression, localization, and function in physiological and pathological contexts. These antibodies are widely used in techniques like Western blotting, immunohistochemistry, and immunofluorescence to investigate PHD regulation in diseases such as cancer, ischemic disorders, and anemia. For instance, PHD2. the most ubiquitously expressed isoform, is a key therapeutic target for conditions like chronic kidney disease-associated anemia, where PHD inhibitors (e.g., roxadustat) stabilize HIF-α to enhance erythropoietin production.
Research using PHD antibodies has also elucidated their roles beyond oxygen sensing, including interactions with metabolic pathways and inflammatory responses. Tissue-specific expression patterns (e.g., PHD3 in adipose tissue) further highlight their diverse regulatory functions. Validating antibody specificity remains essential due to structural similarities among PHD isoforms. Overall, these antibodies provide vital insights into hypoxia signaling and therapeutic development.
×