| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
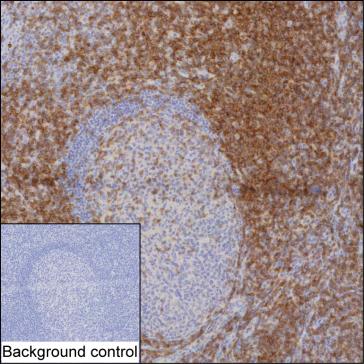
| IHC | 1/100-1/200 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Host/Isotype | Mouse IgG1 |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | Purified recombinant fragment of human CD28 |
| Formulation | Purified antibody in PBS with 0.05% sodium azide |
+ +
以下是3篇关于CD28抗体的代表性文献,按研究领域分类简要概括:
---
1. **文献名称**:*Cytokine Storm in a Phase 1 Trial of the Anti-CD28 Monoclonal Antibody TGN1412*
**作者**:Suntharalingam, G. 等(2006)
**摘要**:该文献报道了抗CD28单克隆抗体TGN1412的I期临床试验结果。6名健康志愿者注射后出现严重的全身性炎症反应(细胞因子风暴),揭示了CD28超激动剂在人体中不可预测的免疫激活风险,推动了药物安全性评估的改进。
---
2. **文献名称**:*CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation*
**作者**:Linsley, P.S. 等(1991)
**摘要**:经典研究阐明了CD28与CTLA-4(均为B7分子受体)在T细胞活化中的拮抗作用。CD28信号促进T细胞增殖和存活,而CTLA-4则抑制免疫反应,为后续免疫检查点疗法(如CTLA-4抗体)奠定理论基础。
---
3. **文献名称**:*Combined CD28 and PD-1 Blockade Expands Tumor-Specific CD8+ T Cells*
**作者**:Hansen, A. 等(2018)
**摘要**:在小鼠模型中,联合使用抗CD28(共刺激激活)和抗PD-1(检查点抑制)抗体,显著增强了抗肿瘤CD8+ T细胞的扩增和功能,提示CD28激动剂与现有免疫疗法的协同潜力。
---
**可选补充**:
4. **文献名称**:*CD28− T cells: their role in the age-associated decline of immune function*
**作者**:Vallejo, A.N. 等(2000)
**摘要**:探讨了CD28阴性T细胞在衰老过程中的积累及其与免疫功能下降、慢性炎症疾病的关系,为靶向CD28的老年免疫调节策略提供依据。
---
**注**:若需扩展或具体领域文献,可提供研究方向(如癌症、自身免疫病或分子机制)。
CD28 is a critical co-stimulatory receptor expressed on T cells, playing a pivotal role in adaptive immunity. It binds to CD80/CD86 ligands on antigen-presenting cells (APCs), providing a secondary signal required for full T-cell activation alongside T-cell receptor (TCR) engagement. This interaction enhances T-cell proliferation, survival, and cytokine production, making CD28 a key target for therapeutic modulation.
CD28 antibodies, particularly agonistic ones, were initially explored to boost T-cell responses in cancer and infections. However, a landmark clinical trial in 2006 (TGN1412) resulted in severe cytokine release syndrome due to uncontrolled systemic T-cell activation, highlighting the risks of excessive CD28 stimulation. This incident prompted stringent safety reforms in drug development and shifted focus toward safer designs, such as monoclonal antibodies with controlled activity or bispecific formats targeting tumor antigens.
Recent advancements aim to balance efficacy and safety. For example, CD28 superagonists are being tested in combination with checkpoint inhibitors (e.g., anti-PD-1) to rejuvenate exhausted T cells in chronic infections or tumors. Additionally, CD28 co-stimulation is exploited in CAR-T therapies to enhance persistence and antitumor activity. Research also explores antagonistic CD28 antibodies to dampen pathological immune responses in autoimmune diseases or transplantation. These developments underscore CD28's dual therapeutic potential—either amplifying or restraining immunity—depending on context and antibody engineering. Ongoing studies prioritize tissue-specific targeting and fine-tuned activation to mitigate systemic toxicity.
×