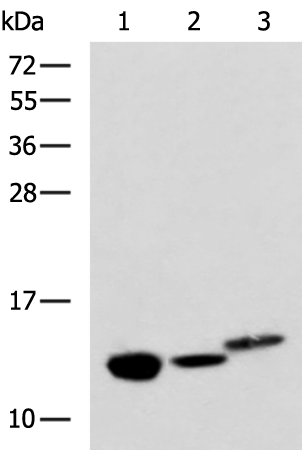
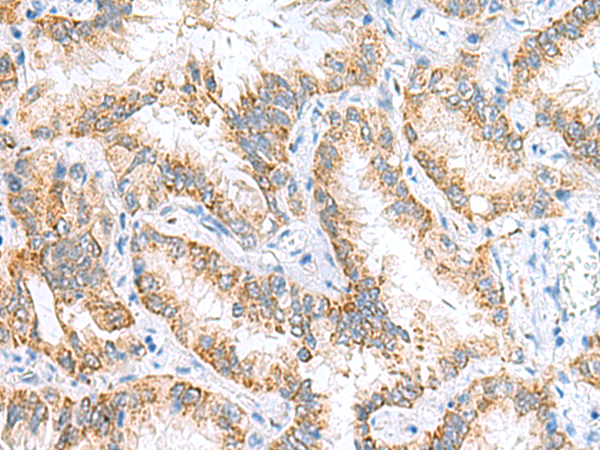
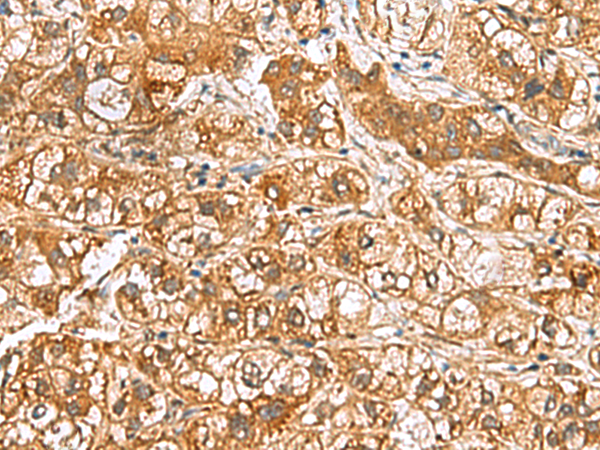
| WB | 咨询技术 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 1/50-1/200 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 1/5000-1/10000 | Human,Mouse,Rat |
| Aliases | PSP; P14.5; UK114; HRSP12; hp14.5 |
| WB Predicted band size | 14 kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human, Mouse, Rat |
| Immunogen | Fusion protein of human RIDA |
| Formulation | Purified antibody in PBS with 0.05% sodium azide and 50% glycerol. |
+ +
以下是关于RIDA抗体的3篇参考文献及其简要摘要:
---
1. **文献名称**:Development of a RIDA®QUICK Immunoassay for Rapid Detection of Gluten in Food
**作者**:Koehler, P., et al.
**摘要**:该研究开发了一种基于RIDA®QUICK技术的快速免疫层析检测方法,用于食品中麸质污染的定量分析。通过单克隆抗体特异性识别醇溶蛋白,可在10分钟内完成检测,灵敏度达5 ppm,适用于食品工业的现场质量控制。
2. **文献名称**:Evaluation of RIDA®QUICK Norovirus Immunoassay for Diagnosing Acute Gastroenteritis
**作者**:Bruggink, L., et al.
**摘要**:研究评估了RIDA®QUICK试剂盒在诺如病毒检测中的性能。结果表明,与RT-PCR相比,其灵敏度和特异性分别为89%和98%,适用于临床快速筛查急性胃肠炎病例,尤其资源有限场景。
3. **文献名称**:Comparison of RIDA®QUICK and ELISA for Aflatoxin B1 Detection in Animal Feed
**作者**:Zhang, Y., et al.
**摘要**:该文献比较了RIDA®QUICK侧流层析法与ELISA对动物饲料中黄曲霉毒素B1的检测效果。结果显示两者相关性高(R²=0.96),但RIDA®QUICK操作更简便,成本更低,适合田间快速筛查。
---
注:RIDA抗体多指德国R-Biopharm公司开发的系列快速检测试剂盒(如RIDA®QUICK)中的单克隆/多克隆抗体,常用于食品安全或病原体检测领域。以上文献聚焦其方法开发、性能验证及实际应用。
RIDA antibodies, developed as part of the Rapid Immunoassay for Dementia Antigens (RIDA) project, are specialized tools designed to detect biomarkers associated with neurodegenerative diseases, particularly Alzheimer's disease (AD). Emerging in the early 2010s, these antibodies were engineered to address the urgent need for early, non-invasive diagnostic methods in dementia research. Traditional diagnostic approaches often relied on post-mortem analysis or invasive cerebrospinal fluid sampling, highlighting a gap in clinical practice.
RIDA antibodies target key pathological proteins like amyloid-beta (Aβ) oligomers and hyperphosphorylated tau, which are critical in AD pathogenesis. Unlike conventional antibodies, they were optimized for high sensitivity and specificity in blood-based immunoassays, leveraging advancements in recombinant antibody technology and epitope mapping. This innovation aimed to enable earlier disease detection, monitor progression, and assess therapeutic efficacy in living patients.
Their development coincided with growing emphasis on precision medicine and biomarker-driven clinical trials. By 2018. RIDA-based assays showed promise in differentiating AD from other dementias in cohort studies. While not yet fully integrated into diagnostic guidelines, they represent a significant stride toward accessible neurodegeneration diagnostics. Current research explores their utility in combination with emerging technologies like digital ELISA and AI-driven data analysis, potentially reshaping dementia diagnostics and therapeutic development pipelines.
×