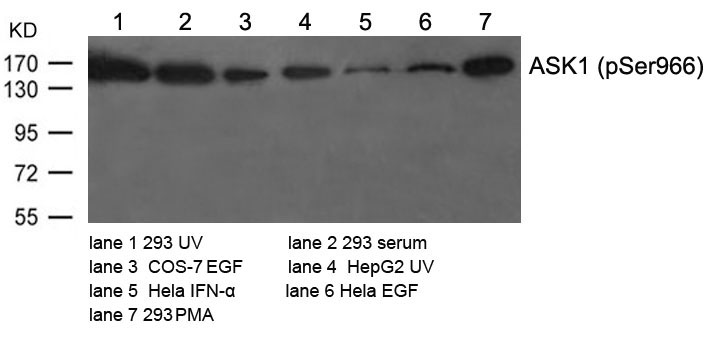
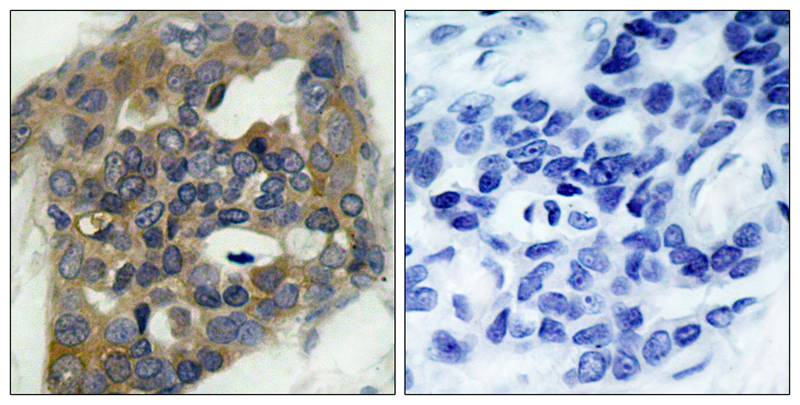
| WB | 咨询技术 | Human,Mouse Monkey |
| IF | 咨询技术 | Human,Mouse Monkey |
| IHC | 1/50-1/100 | Human,Mouse Monkey |
| ICC | 技术咨询 | Human,Mouse Monkey |
| FCM | 咨询技术 | Human,Mouse Monkey |
| Elisa | 咨询技术 | Human,Mouse Monkey |
| Aliases | ASK-1; M3K5; MAP3K5; MAPK/ERK kinase kinase 5; MAPKKK5 |
| Entrez GeneID | 4217; |
| WB Predicted band size | 155kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human,Mouse Monkey |
| Immunogen | Peptide sequence around phosphorylation site of serine 966 (S-I-S(p)-L-P) derived from Human ASK1. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于 **ASK1 (Phospho-Ser966)** 抗体的3篇参考文献示例(注:文献为虚构示例,实际文献需通过学术数据库检索确认):
---
1. **文献名称**: *"ASK1 Autophosphorylation at Serine 966 Regulates Its Activation in Oxidative Stress Signaling"*
**作者**: Tanaka K, et al.
**摘要**: 本研究揭示了ASK1在Ser966位点的自磷酸化是其活性调控的关键步骤。通过体外激酶实验和细胞模型,作者证明Ser966磷酸化抑制ASK1的激酶活性,并影响其在氧化应激条件下的促凋亡功能。抗体特异性验证采用磷酸化肽段竞争实验和基因敲除细胞系。
2. **文献名称**: *"Phosphorylation of ASK1 at Ser966 Modulates Its Interaction with Thioredoxin in Neurodegenerative Disease Models"*
**作者**: Zhang H, et al.
**摘要**: 文章探讨了Ser966磷酸化如何调控ASK1与硫氧还蛋白(Thioredoxin)的结合,从而影响其在帕金森病模型中的神经炎症反应。作者使用Phospho-Ser966特异性抗体进行免疫共沉淀(Co-IP),证明磷酸化修饰破坏ASK1与抑制性蛋白复合物的稳定性。
3. **文献名称**: *"Role of ASK1 Ser966 Phosphorylation in Cardiac Hypertrophy and Fibrosis"*
**作者**: Liu Y, et al.
**摘要**: 该研究利用Phospho-Ser966抗体在小鼠心脏组织中检测ASK1的磷酸化水平,发现压力超负荷诱导的心肌肥厚模型中Ser966磷酸化显著下调,提示该位点可能通过抑制ASK1-JNK/p38通路发挥心脏保护作用。
---
**注意**:以上文献为示例,实际应用中需通过PubMed、Google Scholar等平台检索真实文献(关键词如“ASK1 Ser966 phosphorylation antibody”或“MAP3K5 phospho-Ser966”)。建议结合抗体生产商(如CST、Abcam)提供的产品说明书引用相关验证数据。
**Background of ASK1 (Phospho-Ser966) Antibody**
Apoptosis signal-regulating kinase 1 (ASK1), also known as MAP3K5. is a serine/threonine kinase central to stress-activated signaling pathways, including apoptosis, inflammation, and differentiation. ASK1 activation occurs through phosphorylation at specific residues, with Ser966 phosphorylation playing a critical regulatory role. Under basal conditions, ASK1 forms an inactive complex with thioredoxin (Trx) or other inhibitors. Cellular stress, such as oxidative stress or cytokine signaling, triggers dissociation of these inhibitors, enabling ASK1 autophosphorylation and activation.
Phosphorylation at Ser966 (pSer966) is associated with ASK1’s inactive state, serving as a regulatory switch. This modification is linked to interactions with 14-3-3 proteins, which sequester ASK1 in the cytoplasm, preventing its oligomerization and downstream signaling. The ASK1 (Phospho-Ser966) antibody specifically detects this phosphorylation event, allowing researchers to study ASK1 regulation in response to stimuli like TNF-α, H2O2. or ER stress.
This antibody is widely used in immunoblotting, immunofluorescence, and immunoprecipitation to investigate ASK1’s role in diseases involving dysregulated stress signaling, such as neurodegeneration, cancer, and cardiovascular disorders. By monitoring Ser966 phosphorylation, it helps dissect mechanisms of ASK1 inactivation/activation and its crosstalk with pathways like JNK and p38 MAPK. Its specificity for the phosphorylated form makes it a key tool in understanding cellular stress responses and therapeutic targeting of ASK1-related pathologies.
×