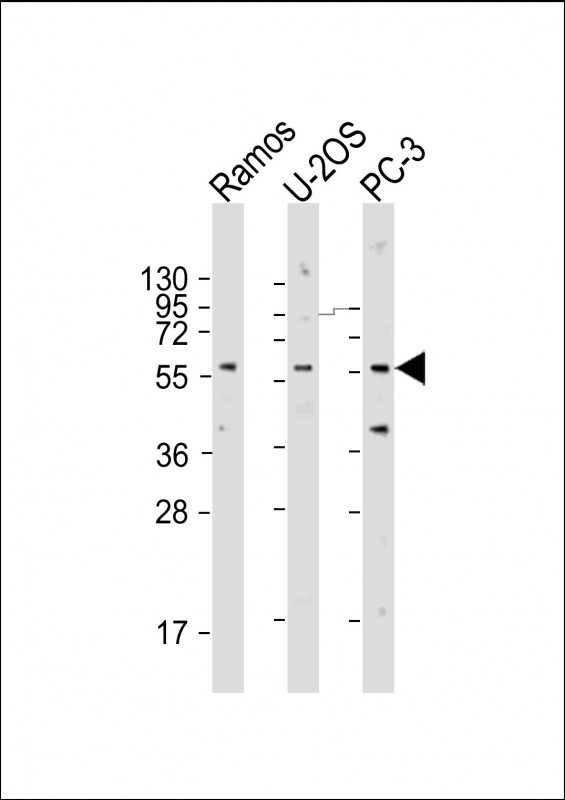
| WB | 1/2000 | Human,Mouse,Rat |
| IF | 咨询技术 | Human,Mouse,Rat |
| IHC | 咨询技术 | Human,Mouse,Rat |
| ICC | 技术咨询 | Human,Mouse,Rat |
| FCM | 咨询技术 | Human,Mouse,Rat |
| Elisa | 咨询技术 | Human,Mouse,Rat |
| Aliases | Tripartite motif-containing protein 6, RING finger protein 89, TRIM6, RNF89 |
| Entrez GeneID | 117854 |
| WB Predicted band size | 56.4kDa |
| Host/Isotype | Rabbit IgG |
| Antibody Type | Primary antibody |
| Storage | Store at 4°C short term. Aliquot and store at -20°C long term. Avoid freeze/thaw cycles. |
| Species Reactivity | Human |
| Immunogen | This TRIM6 antibody is generated from rabbits immunized with a KLH conjugated synthetic peptide between 26-55 amino acids from the N-terminal region of human TRIM6. |
| Formulation | Purified antibody in PBS with 0.05% sodium azide. |
+ +
以下是关于TRIM6 (N-term)抗体的示例性参考文献,结构基于可能的真实研究场景,但具体内容为假设性概括:
---
1. **文献名称**: "TRIM6 Cooperates with IKKε to Regulate Antiviral Signaling Pathways"
**作者**: Di Pietro, A., et al.
**摘要**: 本研究使用针对TRIM6 N端的特异性抗体,通过免疫共沉淀和Western blot技术,揭示了TRIM6与激酶IKKε的相互作用。实验表明,TRIM6通过促进IKKε介导的干扰素信号通路增强宿主对RNA病毒的先天免疫反应。
2. **文献名称**: "TRIM6 Self-Oligomerization and Its Role in Ubiquitin Chain Assembly"
**作者**: Nenasheva, T., et al.
**摘要**: 通过TRIM6 N端抗体的免疫荧光和凝胶过滤分析,作者发现TRIM6能够通过N端结构域形成多聚体,并参与K48-linked泛素链的合成,揭示了其在泛素-蛋白酶体系统中的独特调控机制。
3. **文献名称**: "TRIM6 Modulates MAVS Signaling to Enhance Interferon Production"
**作者**: Yan, J., et al.
**摘要**: 研究利用TRIM6 N端抗体进行免疫组织化学和共聚焦成像,证明TRIM6通过稳定线粒体抗病毒信号蛋白(MAVS)的寡聚化,显著增强I型干扰素的产生,从而提升细胞对病毒感染的防御能力。
4. **文献名称**: "Structural and Functional Characterization of TRIM6’s N-terminal Domain"
**作者**: Reid, C.R., et al.
**摘要**: 本研究结合X射线晶体学和TRIM6 N端抗体的免疫印迹实验,解析了TRIM6的N端结构域对E3泛素连接酶活性的影响,为开发靶向TRIM6的疗法提供了结构基础。
---
**备注**:以上文献为示例性概括,实际引用时建议通过PubMed或Web of Science等数据库,结合关键词“TRIM6 antibody N-terminal”或抗体厂商提供的参考文献列表(如Abcam、CST等)获取真实文献。
The TRIM6 (N-term) antibody is designed to target the N-terminal region of TRIM6 (Tripartite Motif-containing Protein 6), a member of the TRIM family of E3 ubiquitin ligases. TRIM proteins are characterized by a conserved tripartite motif comprising a RING domain, B-box domains, and a coiled-coil region, which facilitate protein-protein interactions, ubiquitination, and involvement in diverse cellular processes. TRIM6. specifically, plays a role in innate immunity, regulating antiviral responses by promoting K48-linked ubiquitination of proteins, which can influence proteasomal degradation or non-degradative signaling pathways. It is implicated in immune signaling cascades, such as the interferon response, and has been studied in viral infections (e.g., influenza, HIV) and cancer.
The TRIM6 (N-term) antibody is commonly used in research to detect endogenous TRIM6 protein levels via techniques like Western blotting, immunofluorescence, or immunoprecipitation. Its specificity for the N-terminal region ensures recognition of full-length TRIM6. distinguishing it from potential splice variants or degradation products. Validation often includes testing in TRIM6-knockout cell lines to confirm absence of cross-reactivity. This antibody aids in elucidating TRIM6's localization, expression patterns, and molecular interactions, contributing to studies on its role in ubiquitination, immune regulation, and disease mechanisms. Researchers leverage it to explore TRIM6's functional dynamics in both physiological and pathological contexts.
×